As is often the case, it’s not a single driver that shapes a trend but rather a confluence of many. Market Drill-Down takes current market data and slices it in new ways to reveal what’s really driving the market.
Things are shaping up to make this the biggest drug launch ever. That’s what the inThought research group is saying about Incivek from Vertex. A new set of oral antivirals—Incivek and Merck’s Victrelis —became available to patients suffering from hepatitis C last May and are quickly changing the face of treatment among the chronic HCV patient population.
We thought it might be interesting to drill down below the surface of the Incivek launch to examine some of the factors contributing to its success—and what the future might hold for a drug that is shaping up to be the first “blockbuster” in a long time. A recent inThought research report cited Incivek sales from its launch in May through the end of September at $495 million, which were based on a Vertex quarterly report. According to inThought, Incivek is the fastest revenue generating drug launch ever, likely to reach $1 billion in under nine months compared to 18 months for Genentech’s Avastin and approximately two years for Abbott’s Humira and Merck’s Januvia.
Incivek’s Perception Advantage
Incivek and Victrelis are alike in many ways: Both have similar mechanisms of action and require the concomitant use of an interferon during and following the completion of their therapy. The way they are being received in the market, however, shows them to be very different. According to inThought, in the same period since its launch where Incivek earned nearly half a billion dollars, Merck reported sales through September for Victrelis of only $53 million. What is it that caused these drugs to diverge so much? For one, their treatment regimens are diverse enough to create the perception that Incivek is more convenient for patients and physicians. Incivek is used with a six-month course of interferon instead of the usual 12 months, while Victrelis requires 12 months of interferons with the possibility of finishing early at six months if the response is good. So, while it may not be all that different in the real world, the perception is six months of interferon with Incivek versus 12 months with Victrelis .
The Market Effect
Let’s now dive into the data to see the effects of these drugs on the market as a whole. Prior to their launches, there was a great deal of hypothesizing among marketers in the HCV arena about “patient warehousing” or patients delaying treatment in anticipation of improved therapy options. A physician survey administered by ImpactRx and inThought found that, on average, physician respondents had 30% of patients not starting therapy, mostly due to the desire to wait for better alternative treatments.
I tapped into the market data, which show total patients filling prescriptions for interferons declined steadily in the 12 months leading up to the May 2011 launches (Figure 1).
That pent-up demand on the part of physicians and patients translated to increased demand immediately following the launch of both Incivek and Victrelis . The launch of the two viral HCV products also has driven up demand for interferon therapy.
Further monitoring of the two launches showed some additional surprises. While Victrelis was available one week prior, by Incivek’s second full week of dispensing, it had posted twice the number of prescriptions as Victrelis . By week six, it had nearly four times the volume. As of the week ending October 14, Incivek’s volume remains at three and a half times that of Victrelis .
At the same time, the share of new patients entering the market has grown from a low of 16% in the months leading up to May 2011, to a high of 30% in August 2011 (Figure 2). It also appears that the patients who are starting therapy are continuing to fill prescriptions based on the refill patterns. While interferon use has increased across the board, Pegasys, the market leader, has seen a significant boost—growing its patient volume 89% from its low in April 2011 to September 2011.
Incivek Defies Expectations
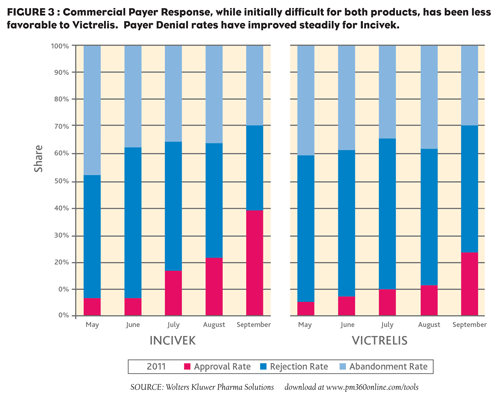
In advance of the launch of both drugs, indicators suggested that payers would steer patients towards Victrelis , partially due to its lower cost per script. Our data show, however, that Incivek fared far better than expected with payers. Despite the rapid growth seen for the two new products, each still struggles with both payer and patient acceptance. In the first three months, the approval/dispense rate for Incivek was 12% of submitted prescriptions and 7% for Victrelis . In the early days, that lost opportunity was split fairly evenly between payer denials and patient abandonments (Figure 3). By September, however, payer response to Incivek had improved dramatically— a 49% approval rate with a 32% denial rate and a 29% abandonment rate. Victrelis , on the other hand, is being dispensed at a rate of 24% with a 47% denial rate.
Although Incivek is more expensive per script than Victrelis , our data suggest that, in actuality, they are very similar (Figure 3). Vertex announced a wholesale cost for the 12-week course of Incivek that is nearly twice the cost as that for a 24-week course of therapy with Victrelis . In considering the patient perspective and their economic reality, we find that overall commercial patients’ out-of-pocket exposure across the entire course of therapy is similar for both products, despite the difference in wholesale cost. While patients’ out-of-pocket exposure for a 30-day supply of Incivek is just over two times that of Victrelis , that out-ofpocket expense will occur over three months compared to Victrelis ’ sixmonth course of therapy.
What Does the Future Hold?
Given the meteoric rise of Incivek, the question inThought and others have asked recently is, “Is this sustainable?” In response, inThought points out that weekly prescription counts show a consistent but stagnant number of new scripts for both Incivek and Victrelis over the past several weeks, and, as a result, it has predicted that sales will level off in 2012 for both drugs. Its analysts, however, also note that “the number of unique writers of Incivek in September was half the number of unique writers of Pegasys, indicating some room for sales growth from doctors who have not yet prescribed Incivek.” Also supporting some room for growth is the fact that, on average, doctors wrote 3.7 scripts of Incivek and 2.7 scripts of Victrelis compared to 5.1 scripts of Pegasys in September.
Only the future will tell, but all indications are that this one is shaping up to be not only great for the patient but the biggest launch ever in terms of revenue. And at least part of its magic is rubbing off on many of the other market players.
FIGURE 1:
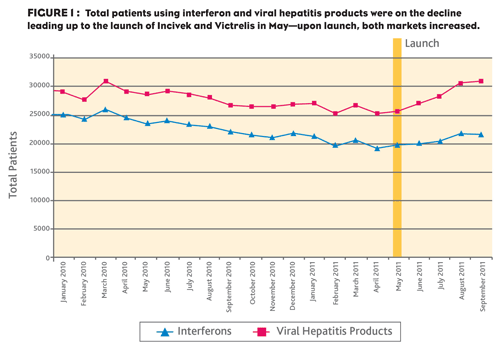
FIGURE 2:
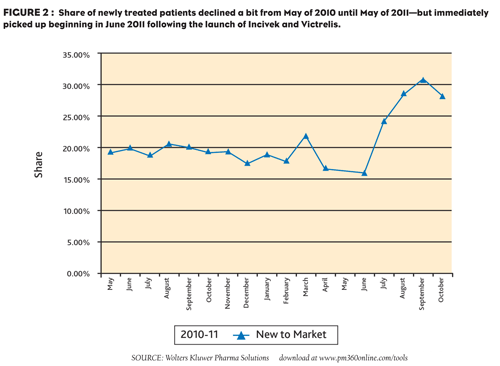
FIGURE 3:
To download Market Drill-Down Tools for this issue’s topic, go to www.PM360online.com/tools
GET THE MARKET DRILL-DOWN EXPERTS WORKING FOR YOU! Submit your marketing question to Wolters Kluwer Pharma Solutions for deep analysis. All queries will be considered for a future column. Email Paula Fullman at Paula.Fullman@source.wolterskluwer.com





