Julie Tibbets 
Public Health Crises and FDA Scrutiny
Every public health emergency or outbreak brings with it a corresponding urgent need for ...
Words & Functionalities Matter: FDA Regulation of Consumer Software and Apps
Today, understanding whether digital health software or apps are actively regulated as medical devices ...
Talking About Your Pipeline
Ahead of commercialization, companies developing drugs and medical devices communicate about their pipeline products ...
Patient Experience Data and R&D
As the U.S. Food and Drug Administration (FDA) works to issue more guidance for ...
Pitfalls of Generic Drug Marketing
While many advertising agencies, companies, healthcare professionals, and consumers alike may not realize it, ...
Exhibit Displays: Beware the FDA
Companies attending large medical meetings to showcase innovative and marketed drug products alike should ...
The FDA, Clinical Decision Support Tools, and the Medical Device Industry
As patients, caregivers, and healthcare professionals rely more on digital healthcare tools and the ...
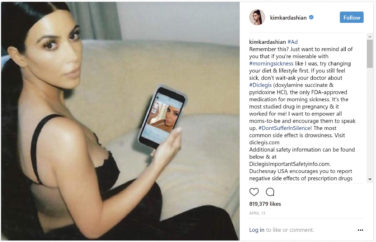
Is Pharma Leading the Pack with Compliant Endorser Disclosures?
In April of this year, the U.S. Federal Trade Commission (FTC) issued more than ...
Disclosing Connections With Advertisers on Social Networking Sites
In April of this year, the U.S. Federal Trade Commission (FTC) issued more than ...
Promoting Products Right: FDA’s Latest Recommendations
Just as the Obama Administration came to a close, the U.S. Food and Drug ...
Laboratory-Developed Tests and the New Administration
On the heels of the recent U.S. Food and Drug Administration (FDA) announcement to ...
Leveraging Patient Preferences and Patient Communications in Device Promotion
As the U.S. Food and Drug Administration (FDA) increasingly focuses on patient engagement in ...
Medical Device Promotion: The Same Rules of the Road as Rx Drugs?
Many pharmaceutical companies today are crossing over into the medical device world in one ...