In April of this year, the U.S. Federal Trade Commission (FTC) issued more than 90 letters to celebrity influencers and marketers of a range of products from clothing and accessories to food and beauty products. Prompted by a request, led by Public Citizen, to take action against noncompliant endorser disclosures that the FTC received last fall, these letters reminded endorsers of their duty to disclose connections with advertisers.
Public Citizen investigated Instagram postings by celebrity influencers and marketers and identified 113 influencers who endorsed a product without an accompanying disclosure of their material relationship with the product or company. Notably absent from the list were pharmaceuticals, despite having safety considerations that arguably heighten the importance of endorser disclosures. So, are pharmaceutical companies the industry leaders in training endorsers about FTC’s disclosure requirements?
The Kardashian Kerfuffle
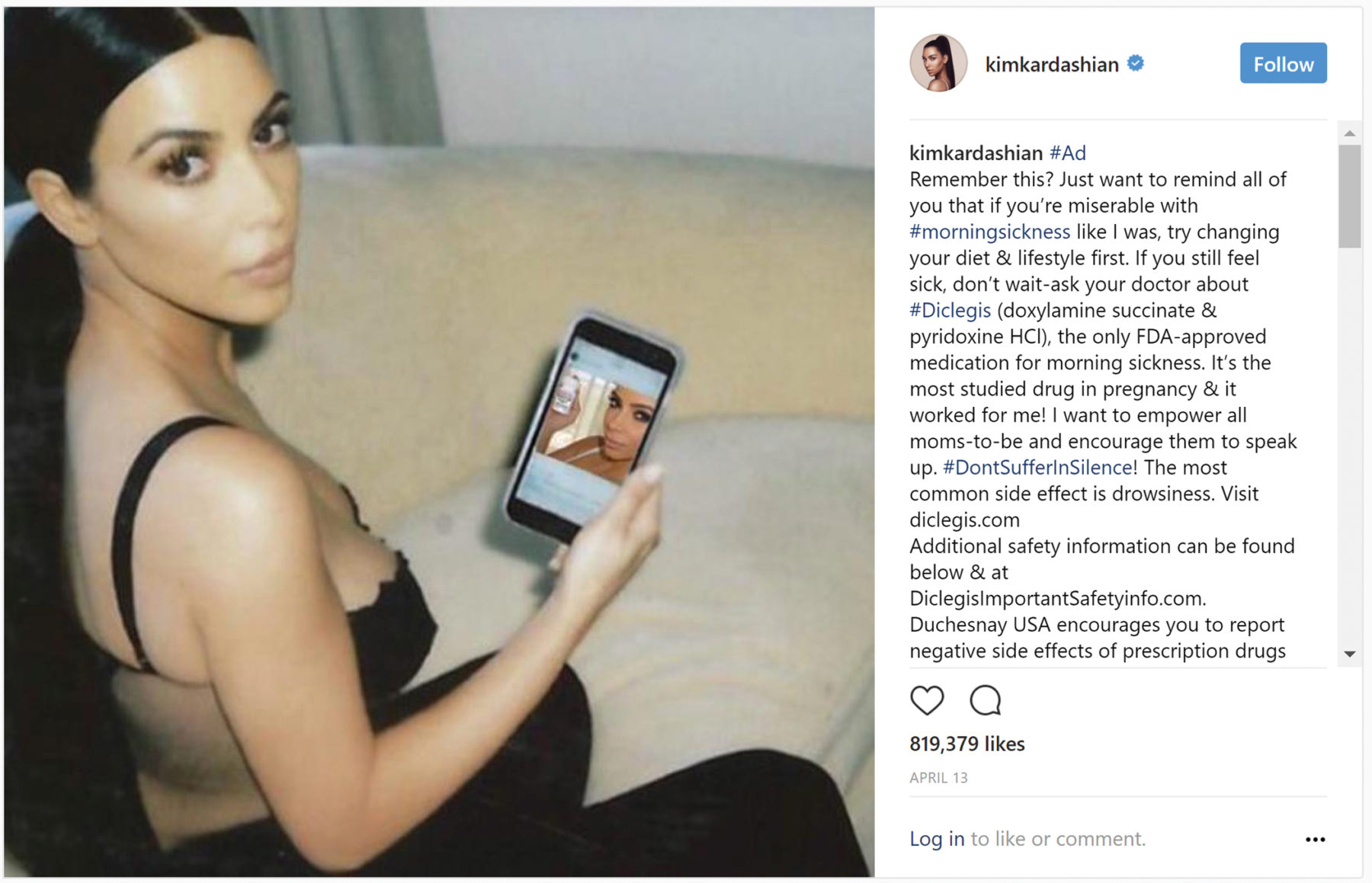
It has been two years since the U.S. Food and Drug Administration (FDA) issued a now infamous warning letter for an Instagram post by Kim Kardashian about a morning sickness drug for omission of risk information, among other things. However, one thing she included in her Instagram post was a clear statement that she was partnering with the drug’s manufacturer to raise awareness of treating morning sickness, disclosing her material connection to the company and product she was promoting.
Just a few months before the FDA issued that warning letter, the FTC issued a guide for industry, The FTC’s Endorsement Guides: What People Are Asking, which includes an entire section on disclosures related to endorsements on social networking sites.
No matter the media, the FTC’s endorsement regulations require that an endorser disclose “material connections” between the endorser and advertiser so that consumers can properly calibrate the weight and credibility they assign to an endorsement. Material connections can include receipt of free products, payments of any kind, and business or family relationships with the advertiser.
Because the advertising and promotion of pharmaceuticals is tightly regulated by the FDA due to the product safety risks associated with those products, pharmaceutical companies navigate a complex set of regulations and legal requirements to successfully market their products. As a result, training of employees, speakers, or others working with a pharmaceutical company to promote a product is necessary to help manage a company’s regulatory risk exposure when noncompliance can trigger significant liabilities and costs for the company.
Endorser statements are no exception and can expose a pharmaceutical company to False Claims Act liability, among other things, which can lead to significant legal defense costs and settlements with the government. As a result, pharmaceutical companies are well versed on training those who speak about their products on regulatory compliance requirements—and it is serving the industry well.