Patients, physicians, payers, and pharma all see Comparative Effectiveness Research from different perspectives. Well-designed trials and good data offer all of them opportunities for better healthcare…even as CER makes them uneasy.
The global healthcare system is evolving into a complex ecosystem where regulatory approval is just a first step toward successful market
access. After approval, biopharmaceutical companies are increasingly seeing their products subjected to health technology assessments (HTAs) by public and private payers. An element of HTA value appraisal is a real-world comparison of a new drug with the existing standard of care, known as Comparative Effectiveness Research (CER). CER is designed to weigh the benefits and risks of various approaches to prevent, diagnose, treat or monitor clinical conditions, to determine which work best for particular populations of patients and in various settings and circumstances.
The likely CER evaluation post approval will require companies to demonstrate the value of their products in the real world, and supplement the traditional demonstration of safety and efficacy through randomized clinical trials. Firms need to view biopharma innovation from a holistic perspective, adapting development programs so that clinical trials address multiple stakeholder demands, using post-marketing observational studies to confirm value, and creating a transparent biopharma valuation protocol based on recognized principles to ensure internal and external validity of the data.
Healthcare stakeholders have myriad, often conflicting goals, including access to services, profitability, high quality, cost containment, safety, convenience, patient-centeredness, and satisfaction, notes a recent paper in the New England Journal of Medicine. 1 Achieving high value for patients must become the overarching goal of health care delivery, with value defined as the health outcomes achieved per dollar spent, asserts the paper.
PLAN EARLY FOR CER
At the earliest possible stage in development, companies should work with CROs to consider how likely their product is to be included in a CER study, how it might perform in such a study, and how it can be best positioned during development. Three approaches can be helpful.
Model to assess the likelihood of the product being chosen for a CER study. This can include an evaluation of ongoing HTA studies—there are currently more than 1,500 ongoing projects conducted by 54 HTA agencies from 24 different countries, including systematic reviews, primary research, clinical guidelines, observational studies and registries—to check whether other products in the same therapeutic category are under review. Key assessment information such as outcome measures, clinical and economic outcomes, final recommendations and agency comments can be tracked in this way.
Of the more than 5,000 studies published to date, the most frequently studied disease areas are cancer, cardiovascular diseases, mental health and behavioral conditions, and musculoskeletal disorders.
Evaluate what form the CER study might take, including study design, study population, the most likely comparators (possibly generics and/or biosimilars) and endpoints, how the product might perform, and who might fund and carry out the study. An analysis of the timing, funding and logistics of previous CER studies would be helpful here.
Adapt early-stage clinical trial protocols to incorporate CER-like elements (for example, an active comparator) to give an early indication of how the product will compare with competitors. Importantly, this early use of CER-like approaches would not delay regulatory submission, and could provide valuable extra information. Such approaches could be used at the following stages: prior to pivotal Phase IIIa trials, where the protocol could be augmented; in post-pivotal Phase IIIb, where a CER-like arm could be added based on those comparators and endpoints that are most relevant to key stakeholders; or post-approval, in Phase IV, where traditional CER elements could be added. Launching a registry could be helpful at this stage.
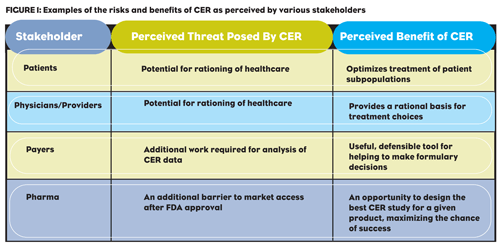
STAKEHOLDER PERCEPTIONS
CER data will only serve its purpose —providing real-world evidence of drug effectiveness—if it is utilized by stakeholders. This will occur if: 1) the threats of CER perceived by stakeholders are understood and addressed, and 2) the presently scattered CER information is centralized, systematically compiled and readily available to decision-makers. One approach to achieve this is to develop a comparative effectiveness index (CEI) to provide comprehensive information at a glance. This systematically compiles data on efficacy/effectiveness, compliance/persistence, safety and health-related quality of life.
Taken together, these approaches will help ensure that the data developed for each product is highly relevant to the constellation of healthcare stakeholders that determine its success once it reaches the market. This can help ensure the timely adoption and financial success of a product.
In today’s post-reform market, CROs are poised to play a central role in incorporating CER information due to their natural touch-points with the full range of healthcare stakeholders throughout the product life cycle. These stakeholder perspectives can be used to develop an appropriate market access strategy including real-world valuation. CROs can apply their regulatory, clinical, and epidemiological expertise to advance CER methods to ensure products are used in a way to optimize patient outcomes.
FIGURE 1:
REFERENCES
1. Michael E. Porter, 2010. “What Is Value in Health Care?” N Engl J Med. 363:2477-2481 (December 23)







