Employing practical experience and real examples, the authors demonstrate the importance of CER to stakeholders and how it may be used now and in the future by pharmaceutical companies to increase market share. Because CER is now firmly embedded in healthcare cost-containment policy, manufacturers’ approaches to CER will determine how successfully they will navigate the new pharmaceutical market
Though pharmacoeconomics has gained acceptance as a central aspect of drug development, approval and adoption, it is still daunting to most non- economists. Comparative Effectiveness Research (CER), focused on the “generation and synthesis of evidence that compares the benefits and harms of alternative methods to prevent, diagnose, treat, and monitor a clinical condition or to improve the delivery of care,” (IOM, 2009) is proving useful in evaluating new medicines and medical treatments. Some pharmaceutical companies have integrated CER into the all-important “Go/NoGo” decision-making process. The American Recovery and Reinvestment Act (ARRA) provided $700 million to fund CER (Conway PH, Clancy C., 2009) and establish the Federal Coordinating Council for Comparative Effectiveness Research. Overall, the U.S. government has invested more than $1.1 billion in CER, which included establishment of the Patient Centered Outcomes Research Institute (PCORI) to evaluate costs, benefits, and outcomes of treatment options.
STRATEGY FOR PHARMACEUTICAL COMPANIES
CER’s “newness” creates an environment of uncertainty for the pharmaceutical industry—with many questions unanswered, chief among them:
“How will CER and PCORI affect my ability to develop, receive approval, and market my drug?” What we do know is this: One of the primary recommendations of the Federal Coordinating Council was to establish a data infrastructure, which would act as a central repository for electronic medical data. Medical information has begun to be collected nationally. The issue is not whether CER will have a positive or negative impact on market share, but whether pharmaceutical companies will react to CER guidelines in a way that increases market share.
The jury is still out on whether PCORI will act like the National Institute for Clinical Excellence (NICE) in the U.S., but there are some rumblings. At this uncertain stage, it is better to be prepared and to be part of the conversation than to hear about the changes well after they have happened. Pharmaceutical companies could be doing two things to prepare for the future. First, pharmaceutical manufacturers can accept that CER is here to stay and will impact future decisions regarding approval, reimbursement, and marketing regulations. Industry has used this type of data in the past to make “Go/NoGo” decisions during early-stage development. However, CER data will now need to be collected throughout development and well into the post-approval stage. Second, the pharmaceutical industry should be part of discussions that will shape the future of CER. AHRQ, AMA, and insurance companies have begun discussing guidelines for the creation of a national clearinghouse for treatment guidelines. These include evidence- based data on treatment options. The pharmaceutical industry should play a larger role in shaping these decisions.
One way companies can use CER to increase market share is to disseminate the results of head-to-head clinical trials. Under a broad definition, CER is currently used routinely in this way to gain approval (by demonstrating superior efficacy) and to gain market share. It is the economic facet of CER that is generally not emphasized when developers seek drug approval or convey key efficacy and safety messages. As policy evolves around CER, this economic facet is bound to play a more prominent role in regulatory and reimbursement decisions.
STRATEGY FOR MARKETERS
Internally, pharmaceutical marketers should be evaluating how CER data fits into their current strategies and tactics. For starters, marketing departments can consider the three opportunities that CER offers. First, marketing departments have been building value stories for their products for many years; this does not need to change. Marketing departments will, however, need to incorporate CER findings into the value story. Second, the 2007 Medicare Prescription Drug Price Negotiation Act requires negotiations between government officials and pharmaceutical companies. In combination with data on cost-effectiveness and evidence-based medicine, drug-makers can use CER data to show how a product provides superior value in the marketplace. In this way, CER can help garner higher prices with the largest payers. Last, marketing departments should consider CER’s role in post-market advertising and promotion. The future of post-market CER data-collection is still unclear, but it is evident that CER will play a more prominent role in these activities and such data will allow marketing departments to reinvent their advertising and promotion tactics. CER scrutiny will certainly put some products, existing or in development, at a disadvantage, but this is where marketers will need to apply their utmost creativity to protect market share and to find and demonstrate value where it is not obvious.
Consider, for example, a recent CER study (performed under a concept devised by AHRQ) designed to help guide appropriate therapy by comparing newer and older oral diabetes agents’ benefits, side effects, and other treatment concerns (Bolen, et al., 2007). The studied agents included second-generation sulfonylureas, biguanides, thiazolidinediones, meglitinides, and alphaglucosidase inhibitors. Newer oral agents (thiazolidinediones and meglitinides) continue to come to market, and it is the responsibility of clinicians and patients to determine whether these new agents compare favorably to older agents (sulfonylureas and metformin). The results showed the need for personalized medicine. A number of conditions (high cholesterol and triglyceride levels, obesity, hypertension, etc.) are prevalent in patients with type 2 diabetes, and a number of common side effects (hypoglycemia, gastrointestinal problems, edema, lactic acidosis, congestive heart failure, etc.) are associated with diabetes medication. The “right medication” was not necessarily the same for all patients with type 2 diabetes; it depended instead on coexisting conditions and medication side effects.
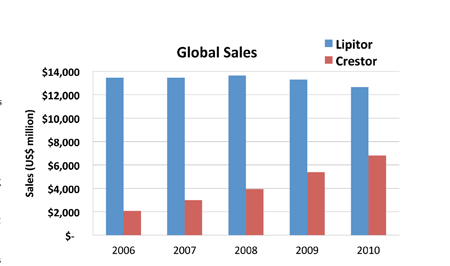
Let’s examine statins, medications used to reduce LDL-cholesterol (LDL-C), as another example where comparative effectiveness was used to gain market share. Lipitor (atorvastatin), a statin that reached the market after four other statins (pravastatin, simvastatin, lovastatin, and fluvastatin) was seen early on in its development program to be more efficacious than previously approved statins. Warner-Lambert saw this opportunity to gain share in one of the biggest pharmaceutical markets and pushed forward quickly with a compelling Phase II study in 1994 and then was granted fast-track (6- month) approval by the FDA in 1997 because they included a study that addressed an orphan disease (familial hypercholesterolemia [FH]) in their NDA submission, along with the pivotal Phase III data in patients with normal high cholesterol. The FH patient population had not previously been studied with other statins.
Lipitor’s clearly superior efficacy helped it gain and maintain the leading position in the statin field. An expanded clinical program helped to continue Lipitor’s lead, including studies such as the “Treating to New Targets” (TNT) trial, which demonstrated unequivocal reduction in LDL- C—and patients on 80 mg of Lipitor had 22% fewer heart attacks and 25% fewer strokes (LaRosa et al., 2010). This data was truly decisive in winning over key opinion leaders in cardiovascular medicine. The TNT results also demonstrated excellent pharmacoeconomic value (Wagner et al., 2009).
AstraZeneca’s Crestor (rosuvastatin) entered the marketplace successfully in 2003, thanks to efficacy and safety profiles similar to Lipitor’s. Additionally, Crestor’s higher potency and lower dose were attractive to patients and prescribers alike. However, Crestor could never catch up with Lipitor commercially, even after the Crestor JUPITER study showed a reduction in overall mortality in patients with normal cholesterol (Hsia et al., 2011). A more recent head- to-head study of Crestor and Lipitor (Nicholls et al., 2011) failed to show significant differences in efficacy. The $205 million investment by Pfizer for co-marketing rights turned out to be a wise move, with Lipitor sales topping out above $13 billion in 2008.
CER-driven demonstrations of real value can help HCPs make better decisions about patient care, help patients see the value in their personalized care, and help payers make better-informed reimbursement decisions. It is clear that a proactive approach to CER will be required for commercial success of new and existing products.
CONCLUSION
This is an obvious win-win situation for prescribers and consumers. However, approximately $700 billion is spent annually on medicine that provides no value (CMS, 2007). An increase in more efficient prescribing will lead to an immediate loss of huge markets that are currently being treated with ineffective medicines.
Most policy wonks and government officials see value in cost effectiveness, which is one of the reasons that consumers fear “rationing.” The recommendations determined by CER will need to be used with caution when impacting patient choice. Not all treatment options work equally well for every patient across the board, nor are they supported by every HCP. The potential for limiting patient and HCP choices is a main concern about the future direction of CER. Clinical trials are biased towards the “perfect target” patient and may not include participants with varying risk factors, comorbid conditions, and “others who have been historically underrepresented” (Garber and Tunis). Payers see CER as a tool that can help guide coverage and reimbursement decisions. This apparent conflict between payers, consumers, and providers has driven Congress’ attempts to draft legislation that both protects patients’ and providers’ rights to choose the best medicine and also allows payers to use CER as a tool for making cost-effective deci- sions. Either way, the regulations will profoundly affect drug manufacturers. In the end, the only way pharmaceutical companies can deal with the new guidelines is to be a part of the debate in a way that their interests help shape the regulations and to learn how to evolve with the changes.
FIGURE 1:
References
Bolen S., Feldman L., Vassy J., Wilson L., et al. (2007). Systematic Review: Comparative Effectiveness and Safety of Oral Medications for Type 2 Diabetes Mellitus. Annals of Internal Medicine. Sept. 18, 2007. 147, 6:386-399.
Centers for Medicare and Medicaid. (2007a). U.S. Centers for Medicare and Medicaid Services, “National Health Expenditure Projections 2007–2017,” p. 3, http://www.cms.hhs.gov/NationalHealthExpendData/Download s/proj2007.pdf
Conway P.H., Clancy C. (2009). Comparative-Effectiveness Research — Implications of the Federal Coordinating Council’s Report. N Engl J Med 361(4):328-30).
Centers for Medicare and Medicaid. (2006a). “Guidance for the Public, Industry, and CMS Staff: Factors CMS Considers in Commissioning External Technology Assessments,” Apr. 11. Retrieved Dec.13, 2011 from: http://www.cms.hhs.gov/mcd/ncpc_view_document.asp?id=7
Chambers J.D., Neumann P.J., Buxton M.J. (2010). Does Medicare Have an Implicit Cost-Effectiveness Threshold? Med Decis Making 30:E14-E27.
Hsia J., MacFadyen J.G., Monyak J., Ridker P.M. (2011). Cardiovascular event reduction and adverse events among subjects attaining low-density lipoprotein cholesterol
LaRosa J.C., Deedwania P.C., Shepherd J., et al. (2009). Comparison of 80 versus 10 mg of atorvastatin on occurrence of cardiovascular events after the first event (from the Treating to New Targets [TNT] trial). Am J Cardiol. 105(3):283-7.
Neuman P.J., et al. (2011). U.S. FDA Modernization Act, SEC- TION 114: Uses, Opportunities and Implications for comparative effectiveness research. Pharmacoeconomics. 29(8):687-92.
Neumann P.J., Weinstein M.C. (2010). Legislating against use of cost-effectiveness information. N Engl J Med. Oct 14 363(16):1495-7.
Nicholls S.J., Ballantyne CM, Barter PJ, et al. (2011). Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. Dec 1, 365(22):2078-87.
Recovery Act. (2011). Recovery.gov: Track the money. Retrieved Dec. 12, 2011 from http://www.recovery.gov/About/Pages/The_Act.aspx
Wagner M, Goetghebeur M, Merikle E, et al. (2009). Cost-effectiveness of intensive lipid lowering therapy with 80 mg of atorvastatin, versus 10 mg of atorvastatin, for secondary prevention of cardiovascular disease in Canada. Can J Clin Pharmacol. Summer 16(2):e331-45.


