With niche launches accounting for an ever-larger share of the market—and most growth—success depends on being able to efficiently locate and motivate smaller, more dispersed audiences.
Traditionally, the pharmaceutical industry has been well-known for its blockbuster drug brands— breakthrough therapies that serve the masses and bring in the big bucks. Today, big-brand therapies are giving way to the introduction of newer, more specialized, pharmaceutical and biotech products to improve people’s health. This growing trend can literally be a matter of life and death for patients and manufacturers alike.
What good is discovering an effective new way to treat a medical condition or disease if you can’t take it to market or let the right people know about it? That’s one of the main challenges that pharma and biotech manufacturers and their marketing teams face each day.
Limited resources are also a big challenge. The economy is still struggling, and even new-product launch budgets reflect that fact. Now, more than ever, every step in the launch process must focus on delivering ROI and minimizing waste.
These are tough challenges no matter what your product, but they are especially difficult when your target audience is in a small niche category. Compared to more mainstream therapy R&D, drug development for orphan diseases starts with scaled-back resources. However, even though the patient population is relatively small, it still takes a lot of resources to do the research, production, packaging, and distribution. The people who need niche products, though, really need them. These products can be vital to the health—and even survival—of some patients.
NICHE DRUG PRODUCTS
Niche medical conditions, or orphan diseases, are defined as a condition that affects fewer than 200,000 people nationwide. These nearly 7,000 conditions (and the patients who suffer with them) are gaining more attention from the pharmaceutical and biotech industries each year.
Currently, there are more than 357 commercialized orphan drugs approved by the FDA under the Orphan Drug Act of 1983. Hundreds more are in the clinical research phases. Of the 30 new drugs approved last year, 11 (or 37%) were for rare medical conditions. That’s the highest percentage on record in approximately 30 years.
According to informedRx, many of the hundreds of specialty medicines likely coming to market in the next 10 years will focus on diseases of aging, such as cancer and neurodegenerative diseases, and on unmet treatment areas such as multiple sclerosis. IMS reports that specialty pharma will be the fastest-growing segment, with an 8% compound annual growth rate.
With this increased niche focus, there are also some significant business advantages. One is that R&D efforts and clinical trials can require fewer patients and can demonstrate significant differences in shorter timeframes. Another is the ability to focus sales and marketing resources on a smaller healthcare provider audience, with fewer sales reps and smaller quantities of marketing materials needed. Orphan drug manufacturers can find greater cost efficiencies in these areas.
Further, the FDA is more interested in approving new treatments that fill unmet patient needs than in helping extend the life of longer-lived brands on the market.
THE PATIENT PICTURE
Some orphan diseases have patient populations of fewer than 100. Collectively, however, they affect nearly 30 million Americans, according to the National Institutes of Health (NIH), which considers these conditions a serious public health concern. Examples of the key areas of orphan research are rare cancers, CNS, endocrinology, immunology, infection, metabolism, and neurology.
Patients and manufacturers alike share a common challenge—insurance formularies. Payers in today’s market don’t readily cover specialty medicines and therapies. It can be highly frustrating when the manufacturer has overcome all other obstacles, the physician goes to write a much-needed treatment for an orphan condition, and the patient is one Rx away from some relief…only to have the patient’s insurance deny coverage. Payer acceptance is one of the final obstacles that must be addressed in taking niche drugs to market.
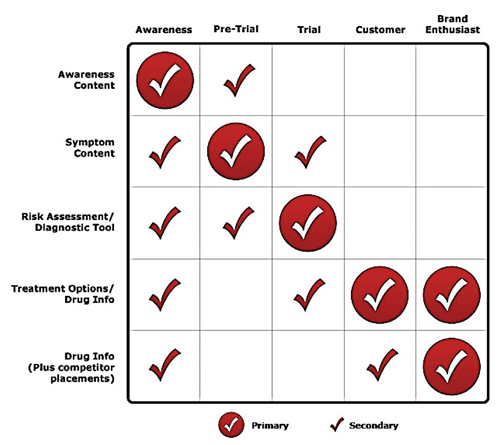
FIGURE 1:
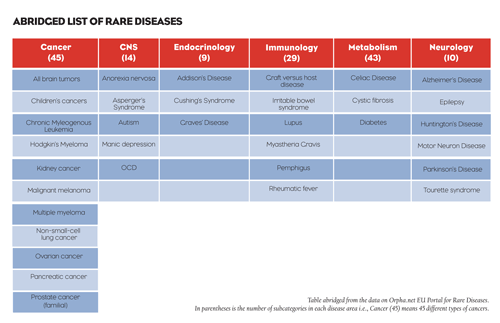
FIGURE 2:

TABLE