Civica Rx Picks First Manufacturer

The new hospital coalition determined to supply the overpriced, indispensable drugs that have remained on the U.S. shortage list has signed its first contract with Denmark-based manufacturer Xellia Pharmaceuticals. It has plans to develop 14 drugs for Civica Rx in 2019. First, it will produce the antibiotics vancomycin and daptomycin. These drugs, the first of the 14 generics to be revealed, are used to treat critically ill patients with serious infections.
“By helping to stabilize the supply of vancomycin and daptomycin, we will have a direct impact on patient safety and public health by providing consistent access to antibiotics that are important treatment options in the management of difficult-to-treat and life-threatening infections,” said Martin VanTrieste, CEO of Civica Rx, in a statement. While the contract manufacturer looks forward to expanding into the U.S. generics market, Civica Rx hopes to buy or build its own manufacturing facilities in the future.
SightGlass Vision Donates Glasses to Children with Myopia
In honor of Global Myopia Awareness Week, SightGlass Vision donated eyeglasses to children in need across the U.S. and Canada. The medical device company develops spectacle lenses to slow the progression of nearsightedness or myopia in children, which has seen a dramatic increase in prevalence over the past several decades. Myopia is now the leading cause of irreversible blindness in parts of Asia and it is estimated that almost half of the entire world’s population, or five billion people, will be nearsighted by 2050. While lenses available in the U.S. correct nearsightedness, they do not stop myopia from getting worse. SightGlass Vision develops new technology to slow myopia progression through early intervention.
Growing DTP Company Receives Exceptional Investment
Health Monitor Network, a leader in DTP and patient engagement for over 30 years, has received significant investment capital from private equity firm WestView Capital Partners to support further dynamic growth.
“Ken Freirich and his team are the exact type of entrepreneurs we look to partner with. They have built Health Monitor into a POC leader and have the vision to maintain that leadership in this strategically important category moving forward,” said Rick Williams, Managing Partner at WestView. “In the point-of-care space, Health Monitor has an unmatched focus, reputation, and track-record of consistent growth. We see a very significant opportunity ahead in this marketplace,” added Jeff Clark, Vice President at WestView.
While the company already has a network of over 400,000 HCPs nationwide that provides award-winning content to millions of patients, HMN continues to add new customers and expand its digital business. This includes new 3D tools and diagrams that have become essential to HCPs in the Health Monitor Network.
Gilead Accused of Withholding HIV Medication
 Allegations filed by more than 140 patients state that Gilead Sciences delayed the release of its safer, next-gen HIV drugs in order to sell out as much of the older generation as possible. A federal judge has approved proceedings after years of filings.
Allegations filed by more than 140 patients state that Gilead Sciences delayed the release of its safer, next-gen HIV drugs in order to sell out as much of the older generation as possible. A federal judge has approved proceedings after years of filings.
The lawsuit claims Gilead kept its older tenofovir disoproxil fumarate (TDF) based drugs on the market since 2004. This is despite the fact that their newer tenofovir alafenamide fumarate (TAF) based therapies were proven safer when the FDA approved Viread, Truvada, Atripla, Complera, and Stribild in 2001, 2004, 2006, 2011, and 2012, respectively. The claims insist that because of the delay, patients who took the TDF meds needlessly developed kidney and bone problems associated with the older drugs.
When Gilead finally did release its newer therapies, it convinced doctors to switch patients over due to safety updates—particularly, its lower risks of kidney and bone toxicities. The lawsuit claims this campaign depended on “the very benefits that Gilead could have and should have incorporated into its prior product designs but withheld from doctors and patients for over a decade.”
The drugmaker has faced these types of allegations before, particularly with its AIDS therapies. However, a spokesperson states “Gilead believes these lawsuits are without merit and we intend to defend against the claims.”
Novartis is paying $3.4 billion upfront and commits potential milestone payments of up to $1.9 billion for Takeda’s (previously Shire’s) Xiidra. Four hundred Takeda employees associated with the drug will also move to Novartis. The drugmaker hopes to bolster its ophthalmic pharmaceutical portfolio with the competitor of Allergan’s Restasis. The drug brought in a total of $400 million last year alone.
“Xiidra, with its unique dual benefits, is an example of the type of innovative advances we invest in for the benefit of patients,” Novartis’ pharma chief Paul Hudson said in a statement. “We look forward to leveraging our well-established commercial infrastructure to bring this medicine to more patients.”
Purdue Accused of Deceptively Targeting Veterans
Purdue Pharma has another lawsuit on its hands, this time from the state of Pennsylvania. The lawsuit claims the drugmaker fueled the state’s opioid epidemic with a deceptive campaign that targeted elderly and veteran patients.
“We are suing the giant, the pharma lord who created OxyContin,” Pennsylvania Attorney General Josh Shapiro said at a news conference. “Purdue owes it to the thousands of Pennsylvanians who lost their lives to [drug overdoses] to stop making excuses and instead to take responsibility for their actions.” He claims the pharma company instructed its sales staff to focus on geriatric audiences. He says it aggressively marketed OxyContin on its website, exitwoundsforveterans.org, while “deceptively assuring veterans that Purdue’s opioids are not addictive.”
The lawsuit chronicles instances in which doctors warned the company that the demand for their OxyContin products was out of control. In one case, the suit claims that a doctor told a Purdue employee that “there is a big abuse problem for Oxy[Contin] and maybe the best thing for him would be if they took his license away.” Purdue continued to call on him for more sales until that doctor was arrested and charged with over-prescribing opiates, it then says. Authorities say nine of the doctor’s patients died of drug overdoses from 2016 to 2018.
The new charges come shortly after Purdue and its owners, the billionaire Sackler family, settled a case with Oklahoma for $270 million. Another case is currently being filed in Ohio and is expected to hit this fall.
AstraZeneca Forges into AI Drug Development
The pharma giant has entered a long-term collaboration with BenevolentAI to use AI for discovery and development of new treatments for chronic kidney disease (CKD) and idiopathic pulmonary fibrosis (IPF).
The AI industry leader has built a biomedical knowledge graph. This is a network base of scientific data (genes, proteins, diseases, and compounds) and the relationship between them. It will be combined with AstraZeneca’s genomics, chemistry, and clinical data and systematically analyzed by machine learning to make connections that humans have not been able to make before. The companies will then work together to interpret these resulting connections, better understanding the underlying mechanisms of these complex diseases. This will hopefully lead to quick identification of new potential drug targets.
The future of CKD and IPF therapies stands to benefit from this method considering their underlying disease biology is poorly understood. This disease complexity requires the interrogation of vast, rich datasets.
Med Device Leaders Develop Afib-Guided Device
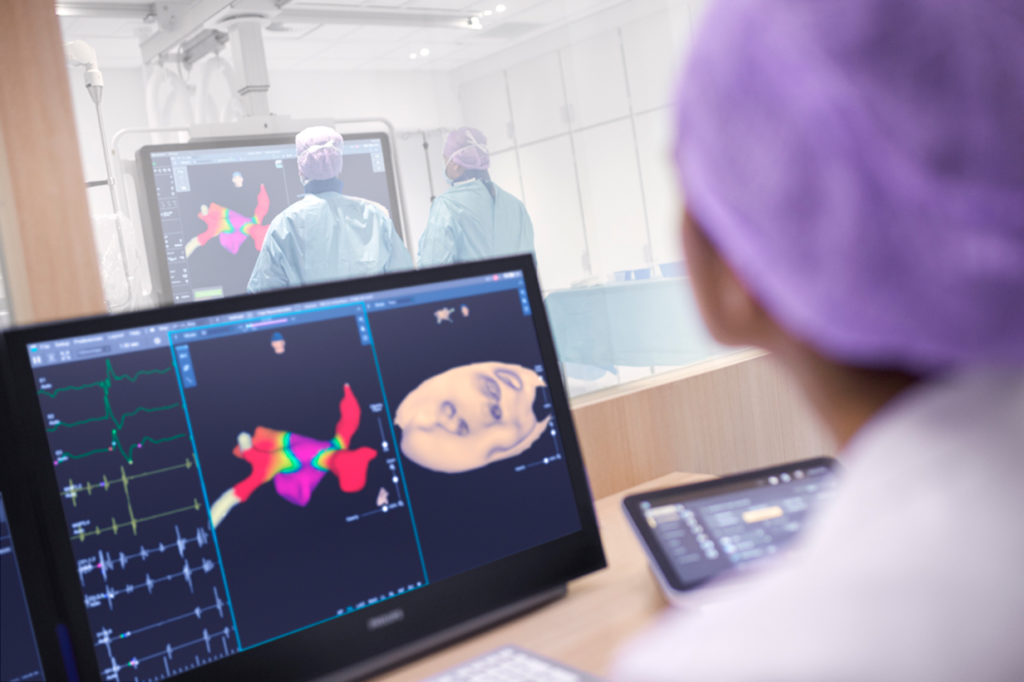
Philips is combining its Kodex-EPD dielectric vision and navigation platform with Medtronic’s line of Arctic Front Advance catheters to create a cryogenic therapy with a guidance system for atrial fibrillation (AF). Their collaborative product aims to reduce the need of X-rays while simplifying navigation during cryoablation procedures. “This integrated solution can guide physicians during the treatment of AF patients with ablation, as they can view detailed, CT-like 3D anatomy,” Philips EPD solutions business leader Marlou Janssen said in a statement.
J&J Launches Cloud Network for Heart Ablation Info
The Biosense Webster heart-mapping hardware and ablation catheters used by physicians around the world can now be brought together through J&J’s new cloud-based CARTONET platform. The idea is to gather and let physicians review data from every heart treatment done with these devices. This data even includes videos and 3D image files obtained during ablation procedures made viewable on any tablet or computer.
“There are thousands of CARTO 3 systems throughout the world containing data that could unlock new clinical insights, refinements to procedures, and personalized education and training,” Biosense Webster Worldwide President Uri Yaron said in a statement. Sharing these statistics and case data between doctors and institutions around the world can be a revolutionary step towards elevating quality of care when it comes to heart procedures.
Condé Nast Hosts First Ever Health Video NewFront
In May, Condé Nast hosted its first ever Health Video NewFront to highlight the company’s success in the health and wellness space. During the event, the company unveiled The Modern Day Health Network, which enables advertisers to buy a full lineup of condition-focused programming developed by “The Script”—a creative team dedicated entirely to health and wellness. Additionally, they debuted Script Studios, a content service that offers branded and whitelabel content for pharma partners looking for deeper integration beyond pre-roll buys.







