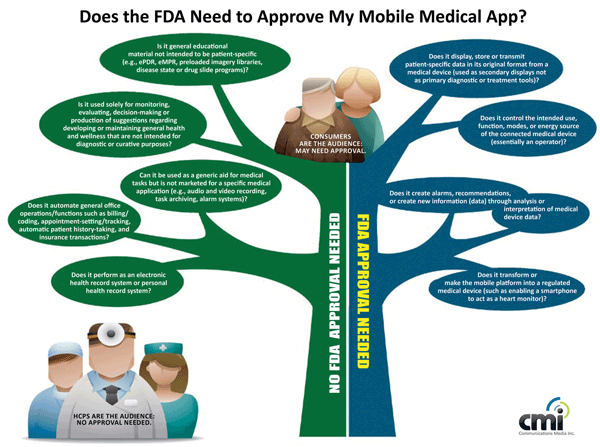
FOLLOW THE BRANCHES
This info graphic from CMI shows that in most cases an app only needs FDA approval if it handles private patient information, is used to make a diagnosis, or acts as or powers a medical device. —Lauren Genovesi
FDA Pleads Innocent in Stalling Medical Innovations
The FDA released a report on the approvals in the year ended September 30, stating that in the past year Americans got access to 24 new medicines before they were available anywhere else. The number of drugs approved was 35, the second-highest number in the past decade, after 37 new treatments reached the U.S. market in 2009. And 22 of the 35 drugs were approved in their first review cycle, according to the report.
The agency report reflects similar findings that the nonprofit advocacy group Friends of Cancer Research put out in June, reporting that new cancer drugs reach the U.S. market several months before they go on sale in Europe.
The FDA used the report in part to refute charges that the government agency bogs down U.S. competitiveness in Pharma and Medical Device industries. Some have accused the agency of driving top companies to Europe, where developers and investors see a quicker path to market.
A second use of the report was to prove that increased user fees from brand-name drug-makers have helped the agency to speed up review times. It stated that these fees facilitated the approval of 34 of the 35 drugs on schedule. The report was released while the FDA was in negotiations with the medical device industry over similar user fees that help pay for product reviews. But device makers have said higher fees in the past have not led to speedier review times.
But FDA commissioner Margaret Hamburg said that swift approval processes are the result of better applications. “The point we’re trying to make is that when high-quality science and good applications come before us, we are able to act swiftly and surely, and we are able to apply considerable regulatory flexibility in our application review process,” she told reporters.
According to the report, about half the drugs approved this year were “significant therapeutic advances” over existing medicines, including seven treatments for cancer and 10 for rare diseases. —L.G.
FDA UPDATE
Approval of Roche’s Avastin was revoked by the FDA for treatment of breast cancer after finding the drug was not safe and effective for that use. Avastin will remain an approved treatment for certain types of colon, lung, kidney and brain cancer.
The FDA approved the first generic versions of Zyprexa (olanzapine tablets) and Zyprexa Zydus (olanzapine orally disintegrating tablets) to treat schizophrenia and bipolar disorder. Generic olanzapine tablets will be manufactured by Dr. Reddy’s Laboratories and Teva Pharmaceuticals; generic olanzapine orally disintegrating tablets will be manufactured by Apotex, Dr. Reddy’s Laboratories, and Par Pharmaceuticals.
The FDA announced Eli Lilly’s worldwide voluntary market withdrawal of Xigris after a recent study showed the drug’s failure to exhibit a survival benefit for patients with severe sepsis and septic shock.
The public was informed by the FDA that a large, recently completed study in children and young adults treated with medication for Attention- Deficit/Hyperactivity Disorder (ADHD) proved no association between use of certain ADHD medications and adverse cardiovascular events, such as stroke, heart attack and sudden cardiac death.
Incyte’s Jakafi (ruxolitinib) tablets were approved by the FDA. Jakafi is a Janus kinase (JAK) inhibitor indicated for the treatment of patients with myelofibrosis, a rare bone marrow disease.
Pacira Pharmaceuticals received approval status from the FDA for Exparel (bupivacaine liposome injectable suspension), a long-acting non-opioid local analgesic for the management of postsurgical pain.
The FDA approved Hemacord, the first licensed hematopoietic progenitor cells-cord (HPC-C) cell therapy and the first cord blood product ever to be approved in the USA. Hemacord is manufactured by the New York Blood Center.
The first artificial heart valve that can replace an aortic heart valve damaged by senile aortic valve stenosis without open-heart surgery was approved by the FDA. Sapien THV is manufactured by Edwards Lifescience. —L.G.



