A New Genomic Test Can Improve Cervical Cancer Testing Worldwide
Rice University is developing a point-of-care DNA-based test for HPV. With HPV testing as the first line of defense when it comes to cervical cancer screening, women in rural areas of the world are the most vulnerable. Cervical cancer, which is preventable and curable if detected early enough, causes more than 300,000 deaths annually in low- and middle-income countries. Currently, doctors depend on lab results for tests done with medical equipment in-office to detect HPV, the most common method being a Pap smear, which is expensive and uses a specific instrument to remove cells from the lining of the cervix to test for HPV causing cervical cancer. Now, Rice University in Texas is in the process of testing an HPV DNA test that is cheaper and more accessible to many, especially women living in rural areas with fewer resources. Screening for HPV is highly effective in preventing cervical cancer because the vast majority of the disease, caused by HPV16 and HPV18, is detectable when precancerous. “We know what we need to do to prevent cervical cancer,” said Kathryn Kundrod, leader of the study and Bioengineer and Postdoctoral Researcher at Rice University. “It’s really a matter of access at this point, and that’s one reason this study is exciting from a global health perspective. It demonstrates a testing process that could potentially be combined with point-of-care diagnostic and treatment technologies to allow women who’ve never had access to be screened and treated in a single visit in settings like a small clinic or a mobile diagnostic van.” Unlike a Pap smear, the DNA test does not require a doctor to use the cervical swab to collect the sample and it only needs small pieces of lab equipment to run. “You could run this test in decentralized locations to detect if somebody has high-risk HPV that causes cervical cancer, and then proceed with diagnosis and treatment,” Kundrod said. The Rice University researchers analyzed self-collected cervical swab samples from patients at the University of Texas MD Anderson Cancer Center and partnered with Hospital Geral de Mavalane in Mozambique to test the accuracy and feasibility of the DNA diagnostic in low-resource settings. While the test’s accuracy is definitely promising, Kundrod and her team are still optimizing it to be faster and more effective in places such as sub-Saharan Africa where many women line up to see HCPs in understaffed clinics and samples may need to be sent out. In the photo above (courtesy of Jade Boyd/Rice University) Kathryn Kundrod (on the far left) is with Rebecca Richards-Kortum and Mary Natoli working in Richards-Kortum’s Rice University laboratory in March 2020.
Sanofi Calls for Innovative Self-Care Ideas
Sanofi’s Consumer Healthcare unit has opened its new Open Innovation Portal, allowing startups, entrepreneurs, research institutions, accelerators, and universities and other supply chain servicers to submit innovative solutions to challenge areas it has identified from creating sustainable packaging to developing new products and technologies. The Innovation Portal is currently focused on facilitating the commercialization of self-care, self-diagnosis, and self-treatment solutions by combining the best external ideas with Sanofi’s marketing expertise, science infrastructure, scale, and consumer reach. Josephine Fubara, Chief Science Officer, Consumer Healthcare, Sanofi, explains, “A problem shared is a problem halved and the Open Innovation Portal is part of our mission to unlock science to bring health in the hands of millions of consumers and patients. The Open Innovation Portal will also serve as an opportunity to partner with the brightest and the best to bring some truly transformative ideas to life for our consumers.” The portal will continue to expand into areas such as digestive wellness, cough, cold and flu, physical and mental wellness, allergy, pain care, personal care, and sustainability. The Open Innovations Portal is a part of the Consumer Healthcare unit’s overall mission to unlock science to empower consumers to take health in their hands, while also meeting sustainability goals of achieving carbon neutrality, reducing waste, protecting ecosystems, and increasing ingredient transparency.
Speeding Up Decentralized Clinical Trials with New Ethics Toolkit
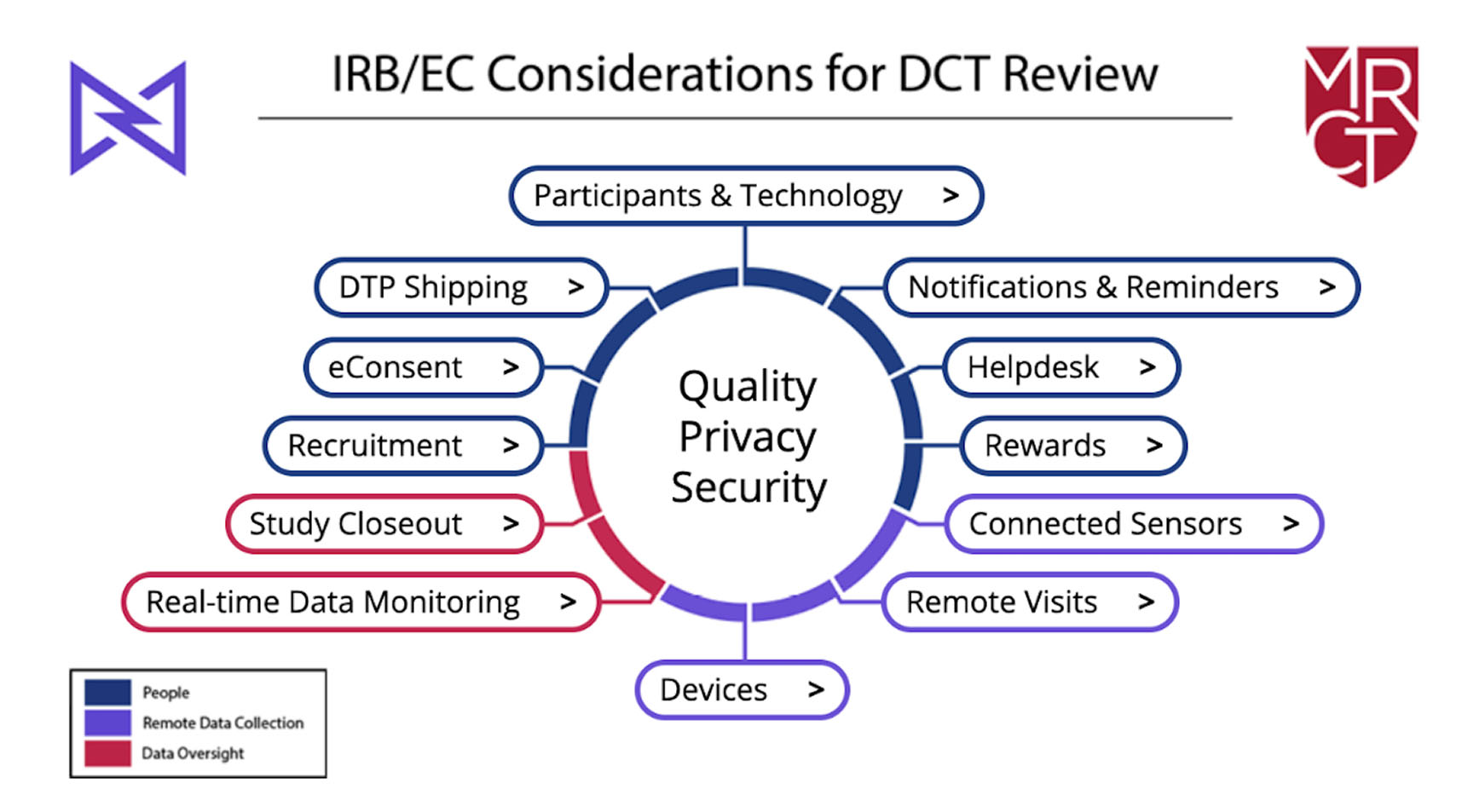
Harvard’s Multi-Regional Clinical Trials (MRCT) Center and Medable, Inc., a leading tech provider for clinical trials, has developed a toolkit to be used by Institutional Review Boards (IRBs)/Ethics Committees (ECs) to standardize and quicken decentralized clinical trial (DCT) ethics review. While decentralized and hybrid clinical trials are being adopted to increase efficiency and accessibility, the process is slowed when it reaches ethical review. The new toolkit provides a common framework, tools, and best practices for uniform ethical review and approval and offers a roadmap for the ethical conduct of DCTs. This standardization and streamlining addresses the IRB turnaround time that has been identified by the National Institutes of Health (NIH) and other industry groups as causing delays that prevent treatments from getting to patients and slow the improvement of public health outcomes. A task force consisting of MRCT Center, Medable, Advarra, the Food and Drug Administration (FDA), the Office for Human Research Protection (OHRP), ethics committees, sites, patients, and patient advocates collaborated to create the new DCT IRB/EC Toolkit, which includes 12 guides organized around three themes of people, data collection, and remote data oversight. The guides also address common elements of a decentralized trial, including electronic consent, electronic clinical/patient-reported outcomes assessment, wearable devices, remote telehealth visits, and others. “The rapid evolution of DCTs have led to ethics review challenges, including lack of familiarity with the varied and evolving technologies, DCT risks, benefits, and ethical issues,” explained Robert Romanchuk, who represented Advarra on the IRB/EC task force. “Additionally, IRB/ECs have different requirements and logistics for submission, review processes, and approval criteria which often leads to variability. It is heartening that other stakeholders have now joined with IRBs to understand their unique concerns with DCTs and have provided guidance and best practices for IRBs, sponsors, and researchers as we move forward in better harmony together.”
Remedy Health Media Rebrands as HealthCentral Corp.
Remedy Health Media is aiming to become the premier destination for patients with chronic and serious illness under a new brand—HealthCentral Corporation. The company’s new brand aligns with its long-term vision and unique commitment to providing medical content and point of care tools to the healthcare professionals who treat patients with these conditions. Along with the rebrand has come a redesign of healthcentral.com, with more streamlined navigation; articles that are easy to read; and more actionable, engaging content, including self-help tools, immersive online experiences, virtual events, and real patient stories to deepen connection to HealthCentral’s patient communities and support patients in living rich, fulfilling lives. The company’s premium patient and professional video and multimedia offerings will come from the rebranded HC Studios as a pioneer in the creation of authentic, real-person health stories. The rebranding is a move under Steve Zatz, MD, who was named Chairman and CEO in late 2022. Dr. Zatz states, “Our new name underscores our commitment to play a central role in helping patients manage and thrive with chronic and serious illness while helping their clinicians deliver the highest quality care possible. It has never been more important to have accurate and actionable information, particularly as advances in medical knowledge happen on an almost daily basis.”







