Social media is delivering commercial benefit to the life science industry in a number of ways (both passively and proactively). At its core, social listening is a powerful and insightful research tool that can help sales and marketing respond more effectively to market forces and drive better customer engagement. Research has shown that listening to social media is a great way to get leading indicators. Where companies are planning a major primary market research initiative, social media listening often helps define the questions to optimize their research before they spend millions of dollars conducting it. In fact, sometimes all the answers can be found in the listening itself, which means a smaller survey can then be used to statistically validate the result.
Perhaps the greatest benefit of social media monitoring is that it plays an important role in compliance and proactive risk management programs. Pharma has been accused of being more hesitant than other industries to develop a digital presence, largely because it is obliged to notify the regulators of any adverse effects that are posted online about its products. But social media monitoring should be seen as an early warning detection system that applies to both opportunities and risks.
It is important for companies to keep a measure of their share of voice and perception in the market—positive or negative. Identifying negative trends relating to the company or its brands early is important as they can be indicative of bigger problems that could occur in several months’ time. Organizations not conducting such social media listening can easily miss key early warning indicators like this and expose themselves to the risk of being seen to act too late in a crisis. While this necessitates appropriate adverse event monitoring, this is particularly important as any adverse events identified could themselves be early indicators of any problems with a brand. As the regulators audit clinical and market research activities ever more thoroughly, having the best possible view of risks by using social media indicators to identify them, is critical.
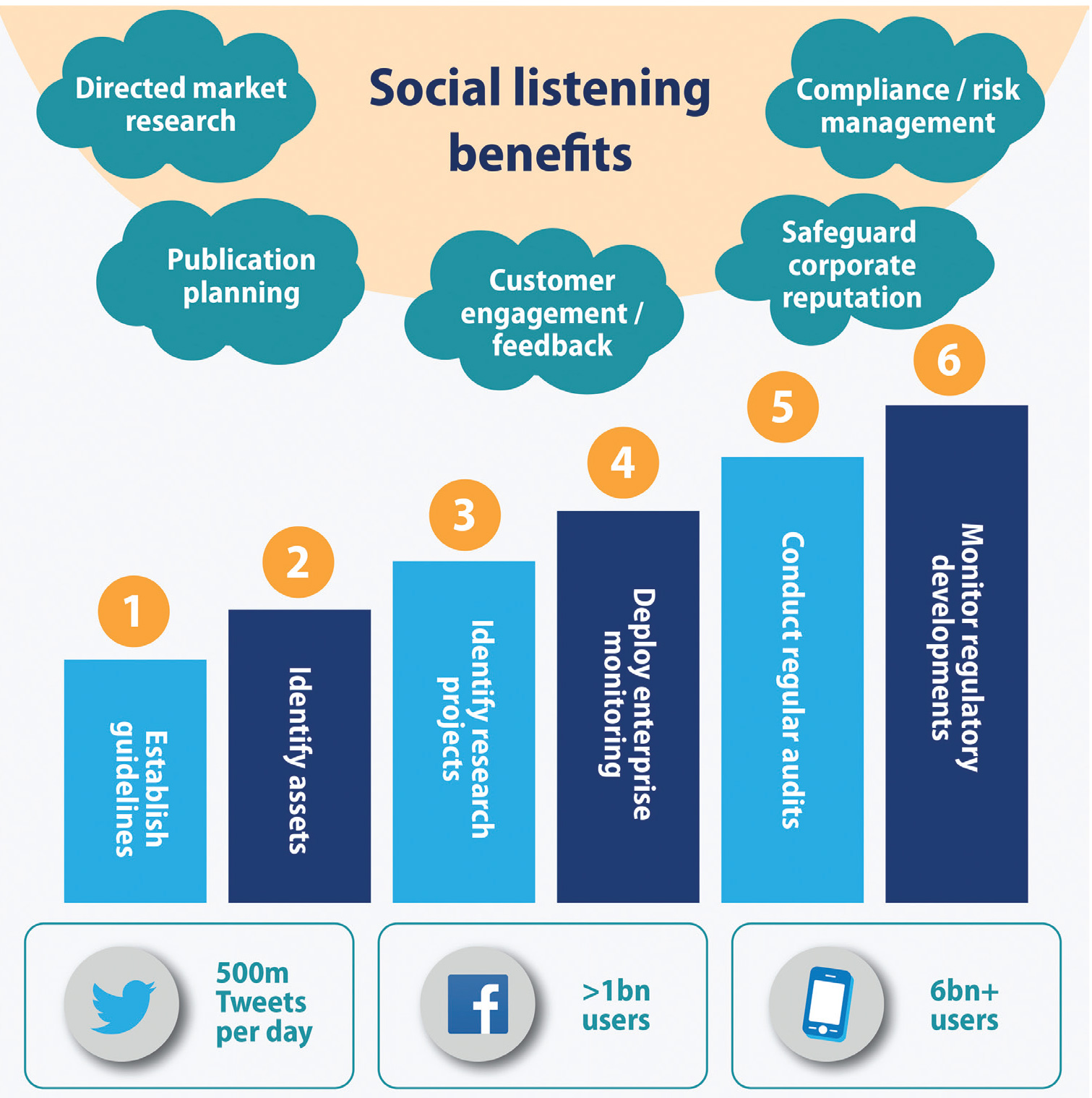
To help companies overcome the inherently gray areas that arise from applying social media properties to the traditional world of regulation, the starting point is to first identify the person who takes ownership of the digital compliance challenge before following a robust, six step process, as follows:
1. Establish and Train on Social Media Guidelines and Best Practices
Even if a company is only listening to social media, clear guidelines about what people can and cannot do are very important. The person or persons responsible for a company-sponsored social media site should know, for example, the company’s policy on what to do if a user mentions an adverse event, asks a question about a product, posts offensive content or discusses an off-label use of a product. In addition, a review and approval process for social media posts by the company should be established to ensure that the company does not inadvertently post inappropriate content. This information should be clearly documented and communicated to employees authorized to engage in social media on behalf of the company.
2. Identify Company-Owned Assets
Social media is global, so creating a channel in one country means it will probably propagate into other countries. One of the top pharma companies recently found that around 100 apps had been created on its behalf around the world. Another company mentioned social media assets that had been created by a brand manager who had since left the company. A full inventory of all your social media assets may reveal some surprises!
3. Identify Social Media Market Research Projects
These are not assets but projects created around the world, where brand managers sometimes ask an agency to do a social listening exercise to find out what is going on. But how do companies know centrally if adverse events are reported in that project? If an issue arises that puts the corporate reputation at risk, regulators may review all available information so it is important to take a regular inventory of all research projects. It is not unheard of for brand managers to subscribe to social listening tools via the corporate AmEx credit card, for example, but they may not be informing the regulators or the pharmacovigilance teams when adverse events are uncovered.
4. Deploy an Enterprise-Monitoring Program
Enterprise monitoring brings everything into one listening program. So if a brand manager wants to do his or her own listening exercise, he has to go through an enterprise-monitoring program that has already been reviewed and approved by the legal and pharmacovigilance departments to minimize the risk. It is similar to how customer-relationship management (CRM) was 15 to 20 years ago. Many brand managers had their own CRM program before it became an enterprise solution with proper controls and monitoring.
5. Conduct Regular Audits of Assets and Programs
Auditing on a regular basis is important because over time people may forget about the enterprise-monitoring program and commission their own programs. For example, a brand manager may create another social media asset or conduct a new piece of market research. Periodic audits should address not only the company’s own assets, but also those of its vendors to make sure they are reporting adverse events.
6. Monitor Regulatory Developments
The FDA’s position on social media is likely an evolving one, as the agency may revise the current draft guidances and issue new ones. In addition, the FDA may issue warning or untitled letters to companies that it believes are not in compliance with its guidance—and such letters may be informative to your own practices. They may also provide clues on the nuances in the FDA’s thinking.
These six steps will not only go a long way in ensuring companies remain compliant but they will also speak volumes about corporate values and integrity in putting patient safety first. In the world of social media—where external perceptions can be magnified hugely via a few clicks from pertinent influencers—such considerations are not to be taken lightly.