Think about the role the hospital plays in the adoption and use of your product before you launch. It can save you from shock and heartache down the road.
When new products crossed my desk for formulary review during my days as a hospital pharmacy director, my first considerations were obviously clinical efficacy and safety, followed closely by the budgetary impact of the product if approved. But, in the back of my mind there was always one additional question: “How much of a hospital product will this be, and why?” In making management decisions, I needed to think through how involved my hospital would be in the overall treatment of patients receiving this product. Would we be initiating therapy and monitoring use of the product, or would those decisions be made primarily outside the walls of my institution? When and where the product is initiated, who is responsible for monitoring continued use, and why that specific product was initially selected matter as much to pharmacy directors as to product managers.
HOSPITAL INVOLVEMENT VARIATIONS
Product managers should ask themselves during early product development, “What role will the hospital play, if any, in the distribution, purchasing, dispensing, and administration of my product?” Chances are there will be some level of hospital involvement for every pharmaceutical or biological product you will market, and that level is also likely to vary significantly for every product you will market, too.
Some products are clearly and simply “hospital products”: their use is initiated, administered, and discontinued within a single hospital visit. In those instances, it is easy for marketers to understand the strategic goal: demonstrate clinical and economic value to hospital pharmacists and hospital-based clinicians to get the product approved on the formulary, then market directly to the specialties treating potential patients so they will order your product when the appropriate patients are admitted. Certainly, the hospital market has a different set of rules than the outpatient setting, but the marketing formula itself is pretty basic.
On the other end of the spectrum exist products that will never see the inside of a hospital, and marketers may launch those products without even considering the hospital setting as an option or concern. Classic outpatient formulary acceptance and managed care reimbursement can be the sole focus of the marketing plans in these instances.
A range of other hospital involvement levels may, however, exist for a pharmaceutical or biological product once launched, and marketers should fully think through how their products may touch the hospital environment prior to launch. Failing to do so may result in some unexpected or even unwanted market realities for which they were not prepared. As with many marketing mistakes of omission, these can be costly.
There is a difference between a product simply being “used” in a hospital and a product being integrally “involved” in the treatment of patients by the hospital for a specific situation. Almost all products will eventually get some use within a hospital, but not every product will be an integral part of the treatment plan for that specific hospital stay. The extent of the product involvement will drive value determinations for the hospital that will, in turn, drive management decisions and even price sensitivity. For that reason, product managers must consider, or even research, the actual role of their products within the hospital setting.
TYPES OF HOSPITAL INVOLVEMENT
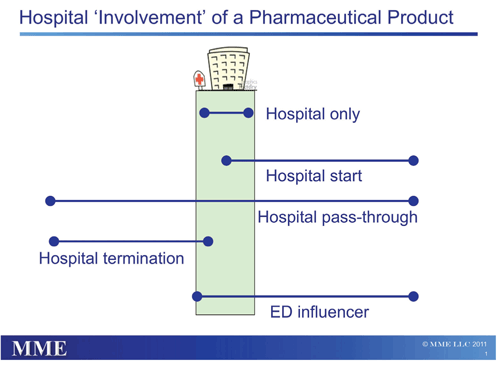
There are five basic types of hospital involvement for a product, based on its use both inside and outside the institution. Notice that each product classification requires a significantly different product marketing strategy.
Hospital-Only. These products are both started in the hospital and ended in the hospital, most often by the same attending physician. Anesthesiology products are a classic example, as well as most IV antibiotics. Marketing must be focused on hospital staff and formulary acceptance, and pricing strategies must ensure product viability with sales limited to this setting.
Hospital Starts. These products are started in the hospital by an attending physician—often a specialist—but the patient continues the therapy after being discharged. This is most frequently an oral medication but can also be an injected or infused product. Marketers often need product acceptance in the institution to create outpatient sales after discharge. For example, a newly diagnosed diabetic inpatient will be started on therapy that may continue for life. Pricing concessions can be considered in the hospital to encourage initial starts, which will help drive additional outpatient sales.
Hospital Pass-Throughs. These products are used by institutions, but product initiation and discontinuation decisions are generally made by clinicians outside the hospital. Often the product is being prescribed for a condition unrelated to the condition causing the hospital admission. For example, patient treatment with hypertension medication may continue unchanged when a patient is admitted for an asthma condition. In these instances, marketing efforts should focus on importance of product continuance during the hospitalization. Minimal contracting may be necessary to allow for unrestricted use, but aggressive price concessions are likely unnecessary.
Hospital Terminations. These products are frequently started in the outpatient setting but stopped as a result of the patient being admitted to the hospital, often because the therapy is no longer controlling the condition. Oral treatments for Parkinson’s disease may fall into this category, for example, if a patient is admitted because more aggressive treatments are required due to disease progression. In these instances, product use may exist in institutions, but the use is really driven by the outpatient setting. Marketing efforts and contracting are minimally important as the institution is not driving product use.
ED Influencers. These products are started in the emergency department (ED) and may continue through the admission and past discharge. For example, anti-platelet medications are often started in the ED after an acute coronary event, and treatment will usually last into the outpatient setting. In these instances, the effect of the emergency room decisions on the hospital and outpatient use should be fully researched and explored. Marketing efforts should focus on the ED physicians but also include the physician specialists, hospitalists, and clinical pharmacists who develop and enforce treatment protocols.
By recognizing the type of the potential hospital product being marketed, marketers can develop strategies for hospitals that are more in line with customer concerns. Understanding the actual role of the product within the hospital will translate into better decisions for marketing, pricing, and contracting to this important segment of our market.