Prevention and treatment of diseases that have seasonal peaks in different regions can be a challenge for public health agencies, healthcare providers, and pharmaceutical and biotech companies. In the United States, a number of diseases—such as allergies, asthma, respiratory syncytial virus, cough, cold, and influenza—occur seasonally, but with onset and peak times that vary by city, state, and region. Manufacturers of influenza vaccine face a marketing environment in which nearly everyone in the United States should be vaccinated, but disease awareness, onset, and spread varies widely by region.
To be successful, vaccine manufacturers must maneuver through an often tricky marketplace with many stakeholders, public and private influences, multiple decision points for brand selection and purchases, and buy-and-bill reimbursement. Influenza vaccine makers face the additional challenge of preparing for, and being impacted by, variability in the disease season and local outbreaks.
INFLUENZA
Influenza is an infectious, contagious disease that affects the respiratory system. The illness can be mild to severe, sometimes resulting in death, with symptoms that include fever, cough, sore throat, runny nose, muscle or body aches, and fatigue. Although it is possible to be sick with the flu at any time during the year, there is a flu season—a time when most flu infections occur.
Flu seasons vary from year to year and locality to locality, in timing, duration, and severity. In general, flu season begins in October and infections peak in January or February, although the season begins in some areas as early as September and other areas may not see a significant increase until late February and March.
FLU VACCINES
To prevent flu, the Centers for Disease Control and Prevention (CDC) recommends that virtually everyone older than 6 months receive a flu vaccination each season. Every year, the Food and Drug Administration (FDA), based on recommendations from the World Health Organization (WHO), decides which flu strains will be included in that season’s vaccines. The WHO, which conducts worldwide surveillance, makes its recommendations according to which strains are most likely to be spread.
The CDC recommends that providers begin vaccinations once the products are available, preferably in the fall, before influenza season begins. Vaccinations typically take place at the same time each season, although with manufacturers shipping their vaccines earlier than in years past, administrations have begun sooner.
Currently, for the 2011-2012 season, there are nine FDA-approved branded flu vaccines, either injectable or nasal spray, manufactured by five corporations. Sanofi pasteur is the largest supplier of flu vaccines for the United States: in 2010, it supplied more than 70 million doses of vaccine, or approximately 42% of the market, with its brands Fluzone and Fluzone High-Dose.
Fluzone Intradermal, approved in May 2011 for patients 18 to 64 years old, will be available for the first time in the fall. It will be the first flu vaccine delivered in a microinjection. With a smaller needle and injected into the skin as opposed to muscle, Fluzone Intradermal will provide a different delivery option for providers and patients.
Novartis supplied the second- highest volume of flu vaccine in 2010: 40 million doses of Fluvirin. GlaxoSmithKline followed with more than 35 million doses (Fluarix and FluLaval). MedImmune, a subsidiary of AstraZeneca, committed to delivering 15 million doses of FluMist, a nasal spray. CSL Biotherapies, in a partnership with Merck, delivered 7 million doses of Afluria. (Please note: The number of doses delivered or committed to was taken from reports by the manufacturers.)
The CDC estimates that 42% of Americans age 6 months and older were vaccinated in 2010, leaving a good deal of the population unprotected—and opportunity for vaccine manufacturers.
MARKETING VACCINES
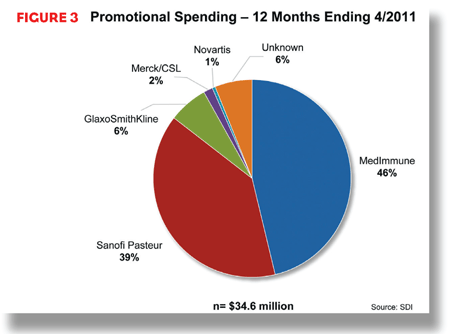
In the 12 months that ended April 2011, flu vaccine manufacturers spent more than $34 million promoting their products. The vast majority of that total—more than 85%— was attributed to two manufacturers: MedImmune and sanofi pasteur. MedImmune led with more than $16 million to promote FluMist. Sanofi pasteur spent $13.6 million marketing its Fluzone brands.
Both manufacturers concentrated most of their promotional expenditures on in-person marketing to providers, through details and meetings. MedImmune focused much of its marketing on pediatricians; sanofi pasteur was more likely to call on primary care physicians. In addition to reaching out to providers, MedImmune invested approximately $1.8 million, or 11% of its promotional budget, in advertising directly to consumers, mostly through Internet and magazine ads.
SELLING MORE VACCINES
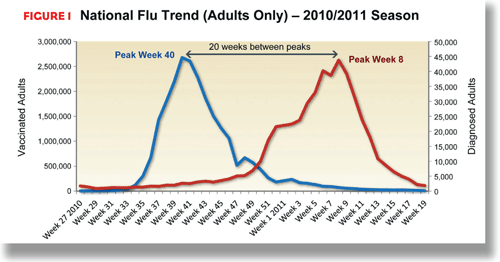
By monitoring geographical differences in season onset and severity, manufacturers can identify additional opportunities. While the vaccination season likely will start at the same time throughout the United States, when and how hard an area is hit with the flu differs. During the 2010-2011 season, vaccinations for adults peaked in the 40th week of 2010 (week of October 3rd). The week with the most patients visiting their physicians with influenza-like symptoms was the eighth week of 2011 (week of February 20th), a 20-week stretch between the peaks.
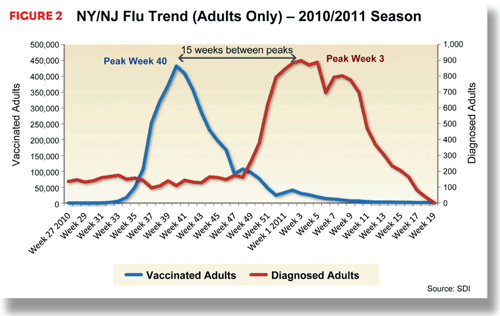
Because regional differences in timing are common with the flu, by monitoring regional trends, manufacturers can identify areas that have yet to hit their peak—places where more vaccine should be sold and administered—and areas where these efforts might be ineffective. For instance, in the New England area, including Maine, New Hampshire, Vermont, Connecticut, Massachusetts, and Rhode Island, flu illness peaked in the 2010-2011 season in the ninth week in 2011, giving manufacturers an additional week of opportunity (21 weeks between peaks). In the neighboring area, largely consisting of New York and New Jersey, the spread of flu began to decrease much sooner, with flu visits to doctors peaking in the third week of 2011.
With information about how the flu is spreading by locality, manufacturers’ strategies for educating providers and patients about the continued benefits of vaccination are more targeted and effective. The flu, its spread and severity by local area, is carefully monitored by a number of interested stakeholders, including health care providers from county health departments to national pharmacy chains, to assess opportunities for increased business and more optimal public health outcomes.



