Over the past decade, the healthcare landscape has changed dramatically with an increased emphasis on engagement to a broader set of stakeholders and an expanded focus on value-based therapies. Most importantly, demonstrating real-world utility beyond regulatory approval has become the new hallmark of success. Medical Affairs is uniquely positioned to address these dynamics as the only strategic function that touches a therapy along the entire product development continuum.
As Medical Affairs continues to expand its role, the breadth of stakeholder interactions and the depth of responsibilities will increase both in number and complexity. Medical Affairs leaders of today must anticipate, shape, and inform the entire healthcare ecosystem.
Novel therapies are increasing in both complexity and cost. Industry headlines are filled with references to access and reimbursement in the context of value-based care and value-based pricing. Value is being defined by an ever-increasing group of stakeholders and decision-makers across the healthcare ecosystem, and measures such as cost effectiveness, patient-reported outcomes, and quality of life are now critical to a drug’s reimbursement, uptake, and utilization. In this environment, successful new product development and commercialization requires the right evidence substantiating value to be available at the right time to the right stakeholders.
The role of Medical Affairs organizations is evolving from disease and product specialists to curators and catalysts of outcomes-focused information. Medical Affairs efforts are fueled by different sources of information that include data from clinical, commercial, and various channels within Medical. This requires a new approach to demonstrate the value of Medical Affairs by successfully operating within an evolved external landscape.
Evolution of the Healthcare Ecosystem
It is clear that the breadth of stakeholders within the ecosystem has grown over the past 5 to 10 years. We also need to recognize, however, that our stakeholders exist within their own individual ecosystems, for example: affiliation with a medical society. To successfully navigate this increased complexity, each stakeholder engagement needs to consider that personal ecosystem. Individual needs and engagement preferences for each stakeholder need to be clearly defined to enable a stakeholder-centric engagement model and ensure success (Figure 1).
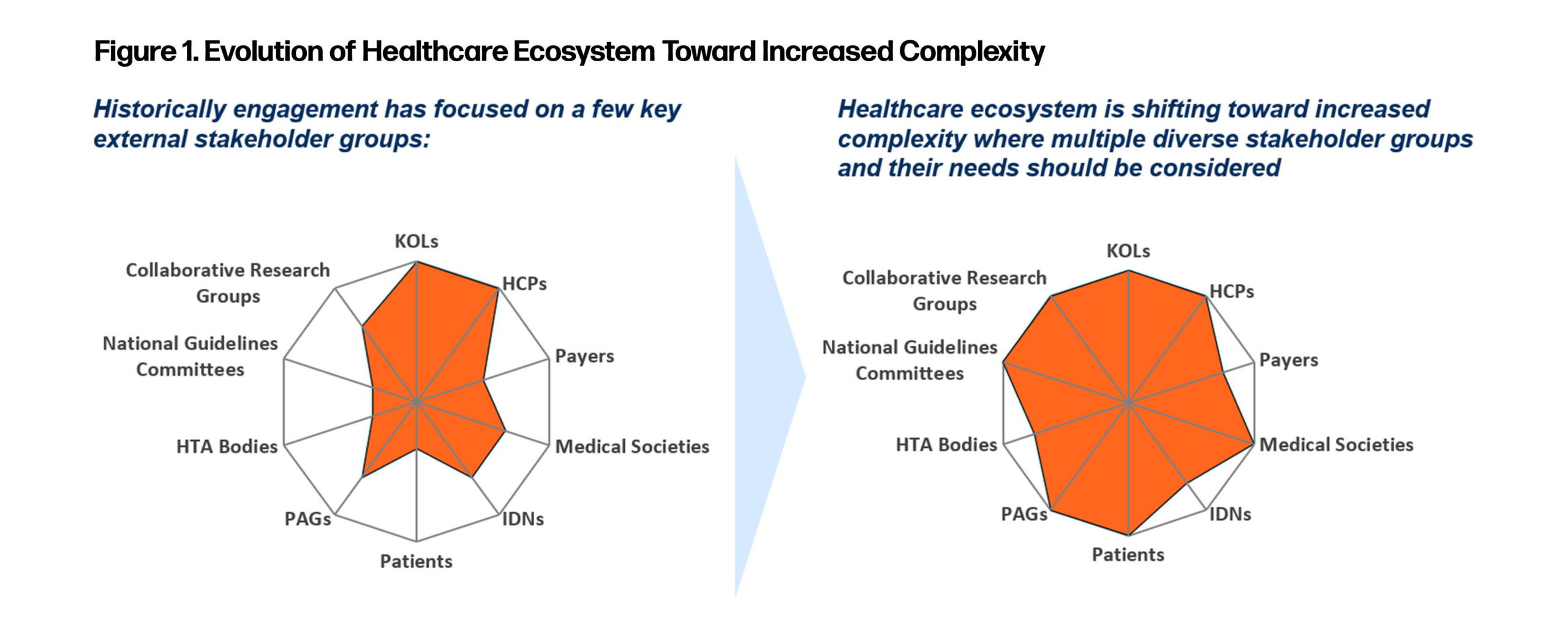 Medical Affairs should be open to using different modalities of engagement for the stakeholders within their ecosystem. It is critical to deliver the right message to the right stakeholder at the right time, leveraging the right channel to ensure maximum resonance and impact amidst the increasingly prevalent noise. Strategically tailoring and differentiating the right message across a multichannel delivery mechanism for each stakeholder will ultimately become the new engagement paradigm.
Medical Affairs should be open to using different modalities of engagement for the stakeholders within their ecosystem. It is critical to deliver the right message to the right stakeholder at the right time, leveraging the right channel to ensure maximum resonance and impact amidst the increasingly prevalent noise. Strategically tailoring and differentiating the right message across a multichannel delivery mechanism for each stakeholder will ultimately become the new engagement paradigm.
Additionally, an overall shift toward more proactive and cross-functional engagement with the ecosystem is altering the fundamental nature of conversations from the perspective of Medical Affairs. This shift, coupled with the rise of digital engagement innovation, has an impact on the overall evolution of the Medical Affairs role, supporting capabilities, and the underlying resourcing model.
Medical Affairs of the Future and Flexible Resourcing
To operate in such an increasingly proactive and cross-functional engagement model, Medical Affairs needs to adopt a flexible resourcing model. Medical Affairs leaders now need to be more agile, be able to scale their organizations up and down, and have flexibility in working with a variety of cross-functional partners, such as translational and market access teams, across different types of programs. Such flexibility and agility affords a faster evolution and growth of the function. A flexible and agile Medical Affairs of the future will also serve as a steward of insights, enabling centralized understanding and knowledge-sharing practices across the organization. The Medical Affairs of the future is well-positioned to be the nexus for overall cross-functional coordination and external engagement with the healthcare ecosystem.
Organizations also need to consider a dedicated C-suite position for Medical Affairs that decides on resource prioritization to effectively support the organization from pre-clinical through launch and beyond. The current profile of a Chief Medical Officer is highly clinically focused and the organizational voice that the role provides is split between the clinical/regulatory outcomes and Medical Affairs. Having a dedicated Chief Medical Affairs Officer is needed to provide a true seat at the table for Medical Affairs and empower it to be an effective strategic partner to the broader organization.
Medical Affairs Value Demonstration Through Data and Analytics
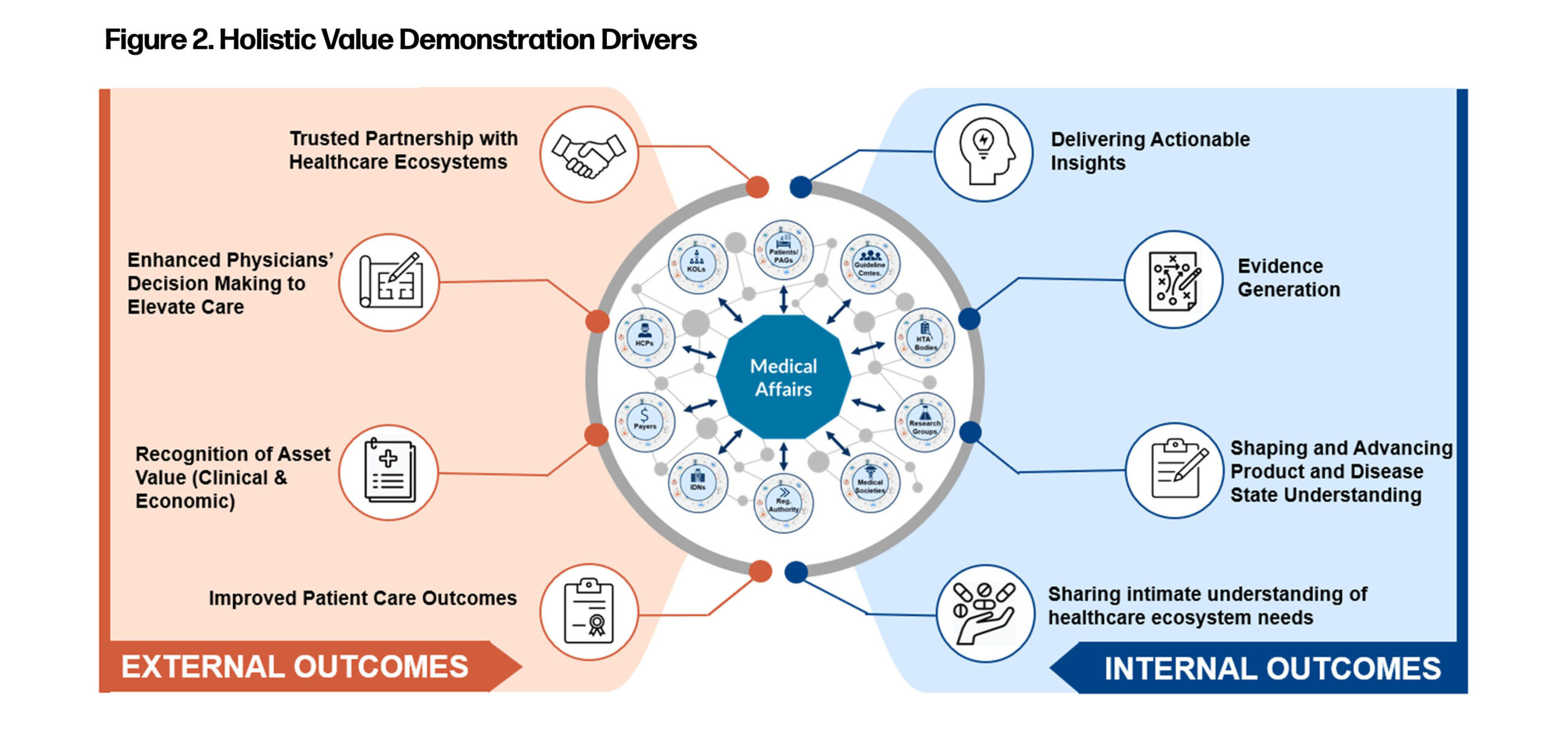
Medical Affairs as a function has been trying to demonstrate value for decades. Organizations need to move away from the quantitative key performance indicators (KPIs) that have existed over the years, and instead look at quantifying the qualitative metrics. Qualitative metrics can serve as a true measure of the contribution of Medical Affairs towards disease state awareness, improvement in stakeholders’ knowledge and confidence in a particular therapy’s mechanism of action, and patient-centered decision-making efforts (Figure 2).
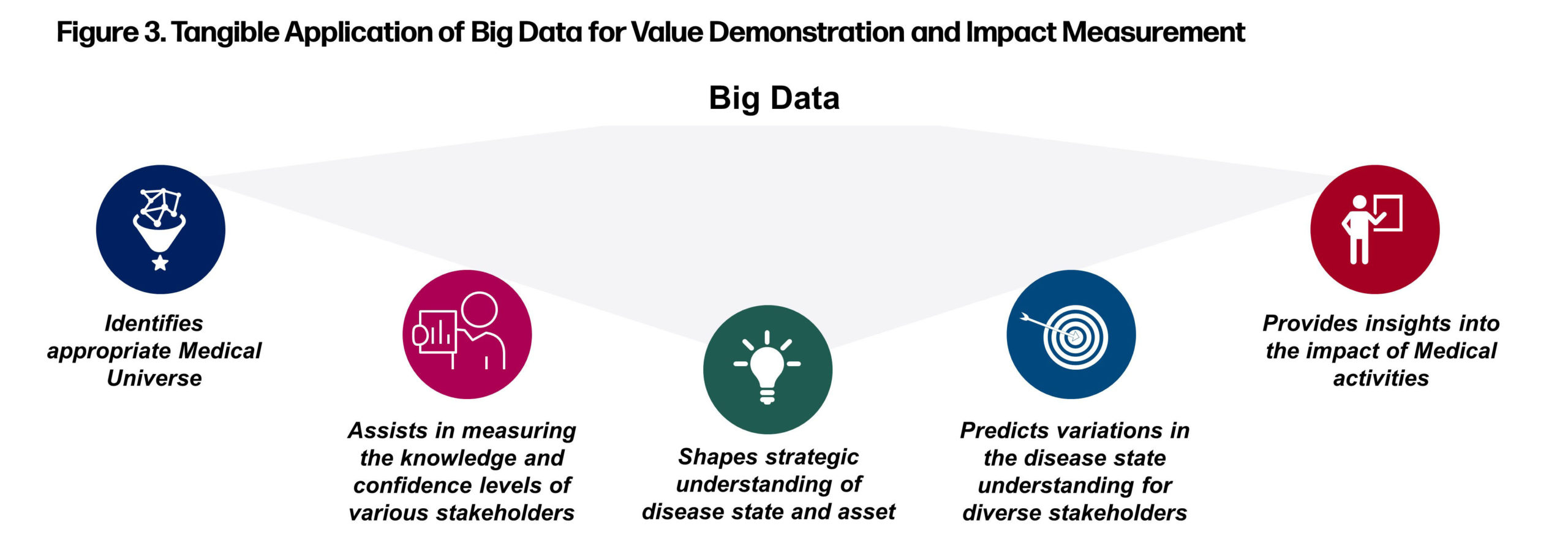
 By embracing the power of big data, Medical Affairs has the opportunity to leverage highly credible scientific information, digital tools, and experiential learning to improve how it interacts with key stakeholders and guide them to navigate the multiplex that the shift to precision medicine has created. Integration of big data with sentiment analysis enables usage of analytical and statistical data modeling techniques to understand the future medical landscape, thereby anticipating changes in disease and competitive landscape and evaluating the impact of current engagement efforts. These insights enable Medical Affairs organizations to establish more effective, efficient, and impactful strategies and solutions (Figure 3).
By embracing the power of big data, Medical Affairs has the opportunity to leverage highly credible scientific information, digital tools, and experiential learning to improve how it interacts with key stakeholders and guide them to navigate the multiplex that the shift to precision medicine has created. Integration of big data with sentiment analysis enables usage of analytical and statistical data modeling techniques to understand the future medical landscape, thereby anticipating changes in disease and competitive landscape and evaluating the impact of current engagement efforts. These insights enable Medical Affairs organizations to establish more effective, efficient, and impactful strategies and solutions (Figure 3).
 In the end, all of this will allow Medical Affairs teams to move away from measurement of activity to overall measurement of impact.
In the end, all of this will allow Medical Affairs teams to move away from measurement of activity to overall measurement of impact.
Integrated Evidence Generation and Dissemination for Impact
In most companies today, evidence generation planning and dissemination planning processes are disconnected, partly because the evidence generation timeline is so long. Evidence dissemination strategy is done too late in the process, and is often only through conferences and papers as data become available. It’s also not done in a stakeholder-centric manner, meaning that different stakeholders need to hear different parts of the evidence narrative in different ways and at different times. Organizations need to consider novel ways to generate evidence beyond traditional clinical trials and long-term follow-up studies to meet the growing scientific data needs of additional healthcare stakeholders. The Medical Affairs leaders of today need to anticipate expanded data needs well in advance of product development timelines and be ready to explore innovate ways to fill these evidence gaps.
These processes need to be intimately linked through an Integrated Evidence Generation (IEG) Framework. Under this framework, both generation and dissemination activities should be proactively aligned under a single cohesive and unified process (Figure 4).
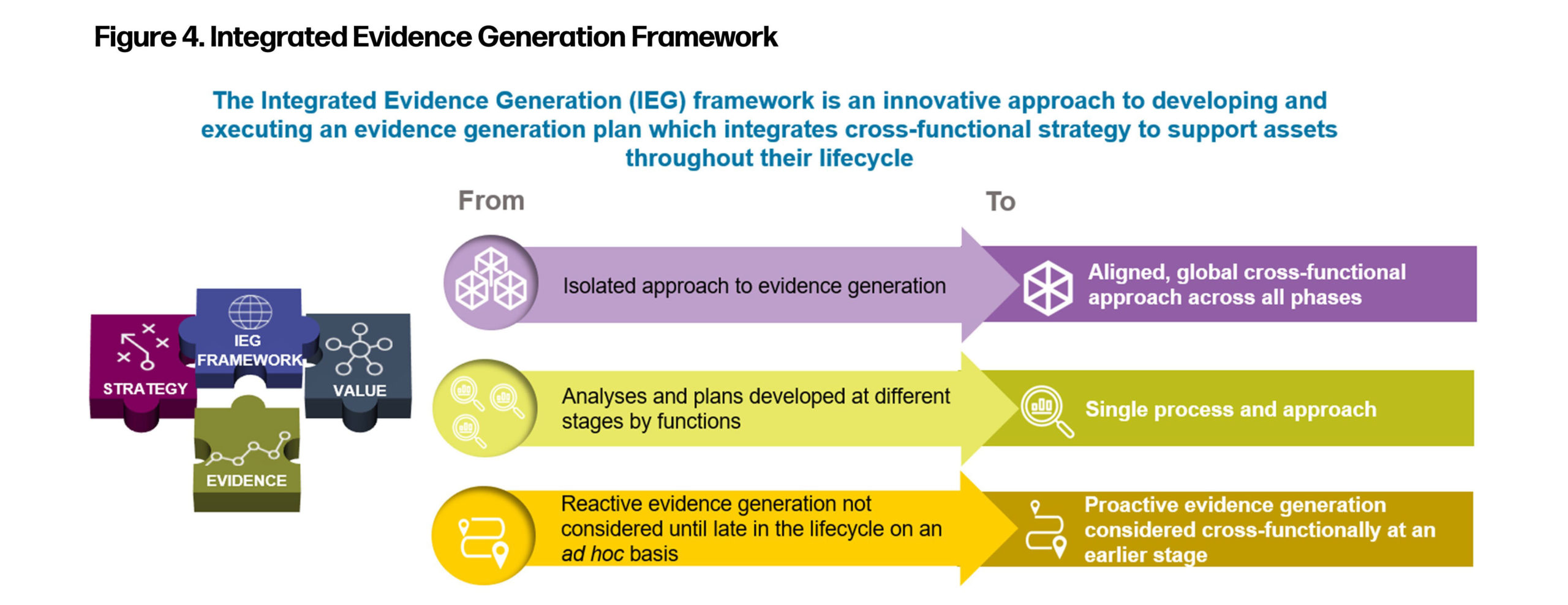 The generation and dissemination planning needs to happen early in the product development cycle, taking into consideration stakeholders beyond regulatory. Stakeholders are now more sophisticated and when the company disseminates their data it needs to carefully consider the who, what, where, when, why, and how.
The generation and dissemination planning needs to happen early in the product development cycle, taking into consideration stakeholders beyond regulatory. Stakeholders are now more sophisticated and when the company disseminates their data it needs to carefully consider the who, what, where, when, why, and how.
In summary, as Medical Affairs adapts to meet the demands of the changing healthcare landscape, techniques used to assess the current and future medical landscapes also need to evolve to understand the needs of all stakeholders from multiple angles. In particular, understanding the evolution of the healthcare ecosystem, considering flexible resourcing, realizing the changing profile of the Medical Affairs leader, and driving the impact and value of Medical Affairs is critical for the future success of an organization.










