Nearly 40% of all the over-the-counter medications in the U.S. were initially marketed as prescription drugs. For drugs to change from prescription to over-the-counter (Rx-to-OTC switch), they have to undergo scientifically robust evaluations by the Food and Drug Administration (FDA). The results are mostly data driven. The medicines that make this switch should demonstrate efficacy as well as a broad margin of safety.1
When intended to be switched to an OTC category, a prescription drug should have the essential properties that can designate it to be self-medicated. The properties that a drug must possess to switch from prescription status to OTC include:2
- A person should easily identify the intended symptom and condition with average intelligence
- The safety margin of the drug should be very high
- The dosage should not be complicated and must be easy to administer
- The drug must be non-addictive in nature
- It must not be a narcotic
- No underlying potentially dangerous condition was experienced by the drug
- The drug should not be for parenteral use
Purpose of the Rx-to-OTC Switch
Product lifecycle extension is one of the most significant reasons why a pharma company considers a Rx-to-OTC switch. When the branded product advances to its patent expiration, competition from the generic manufacturers will increase. The scenario affects the profit unless the pharma company opts for other strategic measures to prolong a product’s life span such as formulation changes, modifying the product drug delivery system, and new indications.3
One can meet most of these options through the 505(b)(2) Regulatory pathway. This is preferred if the company wants to retain the prescription status of the product by extending its exclusivity period and lifecycle. The new drug application (NDA) applied through 505(b)(2) Regulatory strategy includes complete safety and efficacy data.3 This application can also be considered for switching the prescription products to OTC. In case the product does not meet the requirements as per the OTC monograph, the sponsor needs to submit an NDA to switch the product to OTC using 505(b)(1) or 505(b)(2) Regulatory pathway.
Pharma companies with both pharmaceutical and consumer health divisions can opt for Rx-to-OTC switch.3 Changing the product status to OTC helps in maintaining the revenue and growth of the product’s lifetime. The duration of the exclusivity to products new to the OTC market granted by the FDA would typically be three years.
Patients, vendors, manufacturers, and the health industry economy would also benefit from the Rx-to-OTC switch. Lower costs and self-administration of the medication without physician prescription is a significant advantage for patients. If the product had brand recognition earlier, this would benefit the vendors/dealers from having more sales options. It is estimated that billions of dollars are saved by the healthcare systems with the potential use of OTC drug products.3
Apart from the lifecycle extension of the product, an increase in the demand for OTC medication encourages the pharma companies to opt for the Rx-to-OTC switch. The pharma industry would continue to grow as long as patients prefer OTC medications. There is a considerable demand from the end-users for approachable and varied treatment options that are consumer friendly.3
The figure below illustrates the implications of the Rx-to-OTC switch on various stakeholders such as regulatory authorities, consumers/patients, third-party payers, the industry, physicians, and pharmacists.4
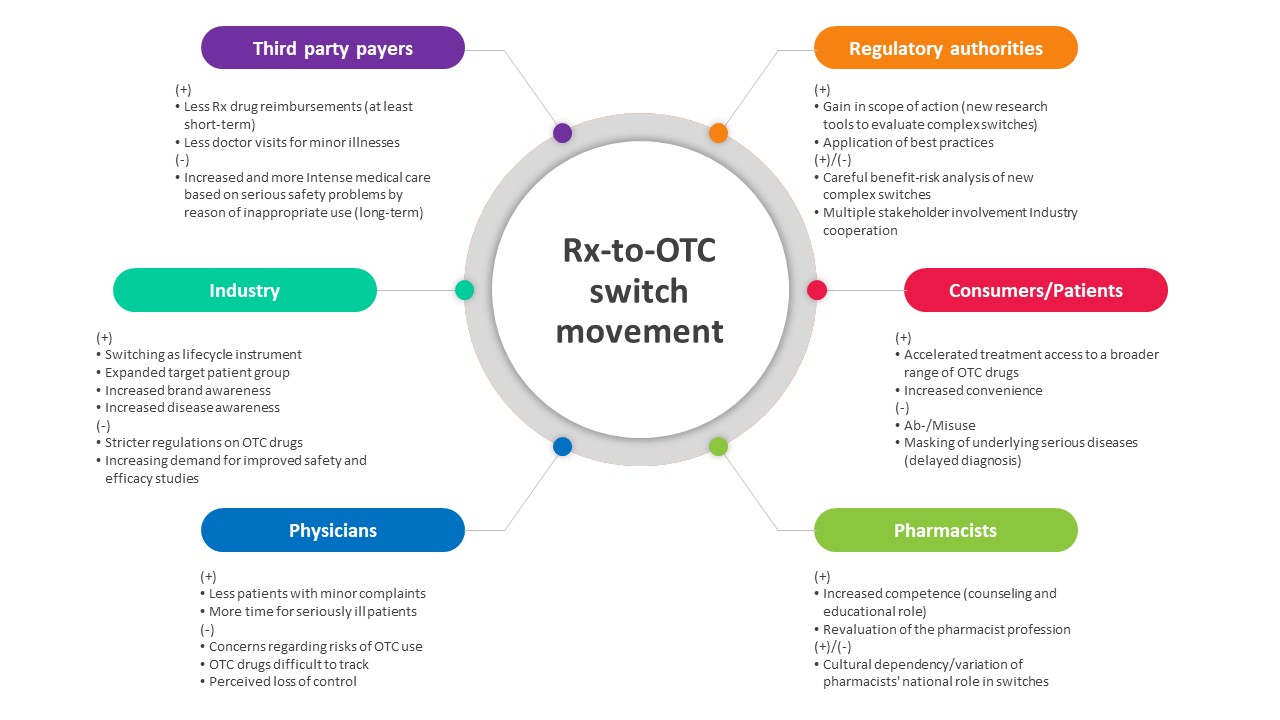 Process/Mechanism Involved in the Rx-to-OTC Switch
Process/Mechanism Involved in the Rx-to-OTC Switch
Before applying for the switch, the sponsors should ensure that the intended product meets the OTC criteria by following the guidelines provided by the World Health Organization (WHO). A product should meet the following characteristics for OTC criteria:2
- During the marketing period, the medicine intended for the OTC switch should have recorded high sales volume implying that consumers have used the product extensively.
- The prescription status of the product must have completed a sufficient period. However, the approved duration of this period varies from one country to another.
- The product’s post-marketing surveillance (PMS) data should not reveal any serious adverse events or safety concerns that increased in frequency during the marketing period.
Under normal circumstances, regulatory authorities consider the safety and efficacy data submitted in the original NDA of the prescription version of the product along with the PMS data. However, the authorities also refer to additional information from the clinical trials conducted by sponsors to support the OTC class. When approving a Rx-to-OTC switch, they can recommend some changes to the prescription version of the product in terms of pack-size restrictions, OTC version approved indications, changes in the label inserts to comply with comprehensible language, etc.2
The other factors that govern the Rx-to-OTC switch in any country are as follows:2
- The literacy rate in the country
- Consumer awareness
- Socio-economic background
- General environment of the individual country
Brief Overview of the Process in the United States
There are two distinctive methods that regulate the U.S.’s prescription and OTC statuses of products. OTC products can be introduced into the U.S. market without FDA pre-approval if the active ingredient, dose, formulation, and indication fall within pre-approved values as implemented in “OTC monograph.”5 However, prescription products or new OTC products require an NDA.
The different ways companies can achieve the Rx-to-OTC switch are:
- OTC Drug Review
- Efficacy Supplement Submission to Existing NDA
- New NDA
The Rx-to-OTC switch would be approved only when the FDA believes that the previous Rx designation is “not necessary for the protection of the public health by reason of the drug’s toxicity or other potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its use, and . . . the drug is safe and effective for use in self-medication as directed in proposed labeling.”2
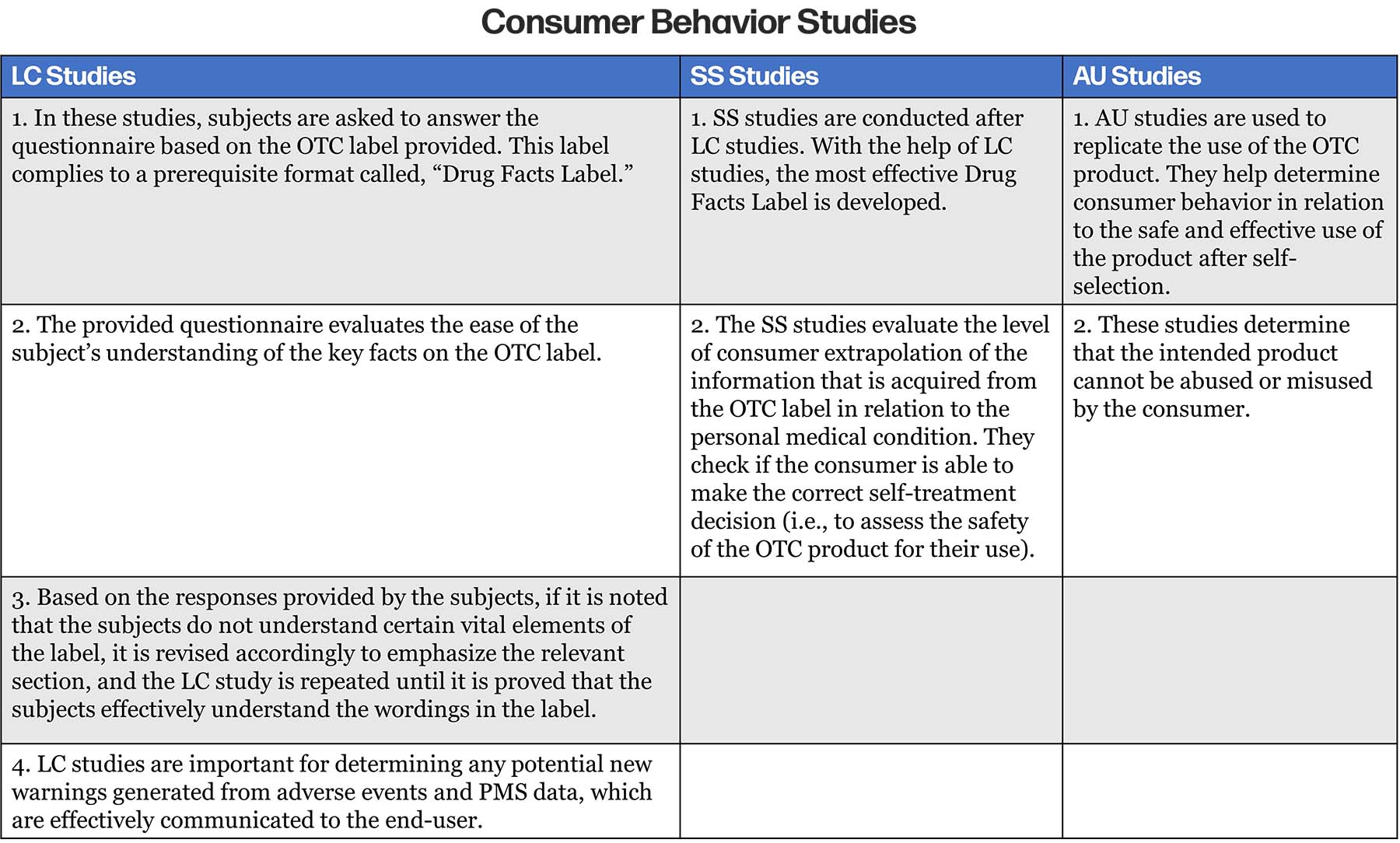
In addition to the data available for the original NDA, the safety and efficacy of an OTC product can be confirmed by conducting Consumer Behavior Studies, which evaluate the use of the intended drug in an OTC setting.2
Consumer Behavior Studies comprise of the following:2
- Label Comprehension Studies (LC Studies)
- Self-Selection Studies (SS studies)
- Actual Use Studies (AU Studies).
Details of each study process are summarized in the below table2:
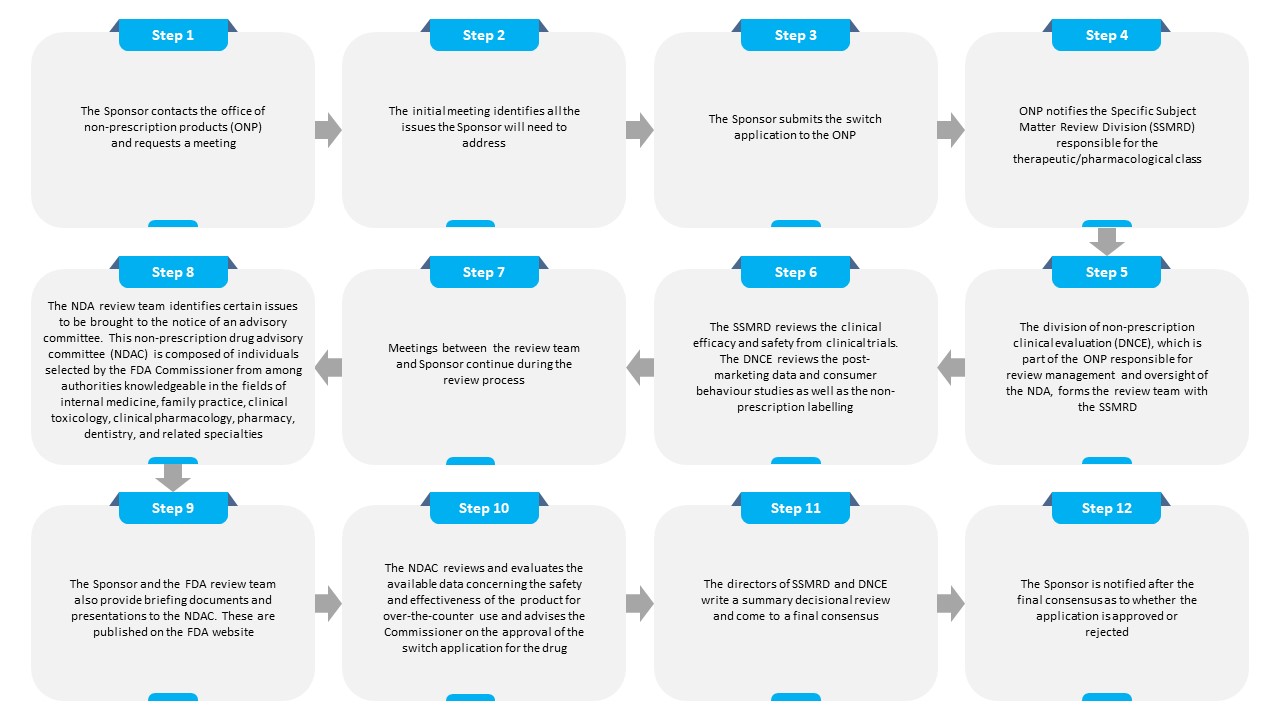
 Summary of the RX to OTC Procedure
Summary of the RX to OTC Procedure
The complete process of switching a product from Rx to OTC in the U.S. is summarized below:
 Conclusion
Conclusion
The FDA’s decision to switch a drug from prescription status to an OTC status is based on the benefit vs. risk analysis of the intended drug product. The contributing factor for benefit-risk analysis is the ability of the individual/consumer to accurately identify and self-diagnose the symptoms of the condition. To summarize, the Rx-to-OTC switch should be based on a thorough and transparent scientific assessment of the relevant data. If successful, this switch will contribute to the enrichment of public health by raising disease awareness and encouraging individuals to take up the responsibility of their health and manage their own disease status rather than relying on the burdened healthcare system.
References:
1. Saleh. N. “From Rx-to-OTC: 13 drugs that made the switch in the past decade.” MDLinx. 2020. Available at: https://www.mdlinx.com/article/from-rx-to-otc-13-drugs-that-made-the-switch-in-the-past-decade/5ZAzuyvaSt4DJ5RMMt2nkU. Accessed on 16 Apr 2021.
2. Kartha S.S., Kulyadi G.P., Bhat K., et al. “Switching drugs from Rx to OTC status-a regulatory perspective.” Journal of Young Pharmacists, 2017; 9 (1), pp. 3-7.
3. Sengupta G, Rein Am. “Rx-to-OTC Switches: Growth, Drivers & The Role of Patients.” LLS Health Technical Team. 2020. Available at https://www.lubrizol.com/Health/Blog/2020/11/Rx-to-OTC-Switches. Accessed on 16 Apr 2021.
4. Erlangung des Doktorgrades. “Rx-to-OTC switch and the provision of data exclusivity in Europe – specification and elaboration of eligibility criteria based on a status quo analysis.” Dissertation. Submitted to Fachbereich Pharmazie der Philipps-University at Marburg. 2013
5. USFDA. (2020) Drug Applications for Over the Counter (OTC) Drugs. [Internet]. 2020. Available at: https://www.fda.gov/drugs/types-applications/drug-applications-over-counter-otc-drugs. Accessed on 20 Apr 2021.






