Marketers who want their new brands to excel need to choose KPIs that go beyond the conventional metrics of sales, share, and growth. Build the right combination of leading and lagging indicators to define and track launch success, and show instantly where to make changes to correct the launch trajectory.
In a world where pharmaceutical launches have just a few short months to make their marks, companies are under pressure to maximize uptake with very little margin for error. Yet, fewer new brands are succeeding in gaining significant market share. Diligent and early preparation is the key to ensuring an optimal course. Going forward, marketers who excel will have well-designed leading and lagging key performance indicators (KPIs) at the center of their global launch programs. These metrics will enable full alignment across all activities, visibility of progress towards strategic goals, and the ability to resolve deviations from plan in a timely and effective manner. Those who fail to optimize KPIs in a best-practice framework run the risk of sabotaging years of research as well as millions of dollars in commercial investment.
The growing importance of KPIs comes as R&D is yielding fewer innovations, patents are expiring in increasing numbers, and maximizing the value of every product launch is imperative for long-term success. Adding to the pressures are major shifts in stakeholder power and, in the launches themselves, changes in focus from primary care to specialistled and niche therapy areas. Approaches to market are also more complex, with the increasing pursuit of multiple indications, expanding presence in “pharmerging” economies1, and healthcare reforms bringing new considerations for market access and global launch sequencing.
Significantly, too, new products must do well in a very short period of time. Rarely can those that start poorly significantly improve on their trajectory in the first six months; long-term performance may even be determined in as little as three months from launch. With the growth contribution of launches falling and fewer new products capturing significant market share, ensuring optimal readiness and flawless execution is essential.
Despite the imperative to succeed, less than 1% of new brands excel in today’s pharmaceutical environment. Over the course of three launch excellence studies, IMS analysis of thousands of new brands across 90 therapy areas and eight different countries in a 10-year period2 reveals that many companies still fail to deliver on the four fundamentals for success:
- Align and prepare well for the launch.
- Build a powerful and pertinent value proposition.
- Engage all stakeholders effectively.
- Define and track launch success.
From a launch preparation perspective, our experience has shown that alignment, visibility and quality of execution—while essential to planning an optimal course—are significant issues for many organizations. Internal misalignment, lack of clarity around critical success factors, and poor visibility into launch performance are common; these errors result in functional silos instead of cross-functional teams, major disconnects around strategic goals and critical gaps in understanding. Without a structured, systematic approach to monitoring and managing the progress of launch preparation and execution, companies continue to run the risk of turning potentially winning brands into also-rans.
WHAT ARE KPIs?
“You can’t manage what you don’t measure.” This adage may be old but it remains as true as ever in the context of pharmaceutical launches. Given the need for complete alignment and concord around the strategic goals for the launch, all companies can agree on the importance of KPIs in defining a successful outcome. Our analysis has confirmed that proactively establishing metrics and closely monitoring performance both pre-and post-product launch are central to ensuring an initial strong trajectory—the ultimate key to long-term success.
While hundreds of performance indicators and thousands of metrics are possible, the phrase “key performance indicators” shows the way. By their very description, KPIs focus on only the most important and pertinent metrics. They must be crucial and relevant to the organizational or brand strategy, be actionable for managing brand performance and initiating positive change, and—when measured against specific criteria or thresholds— yield insights towards the desired objective.
WHY ARE KPIs NEEDED?
Every product has its own unique success factors, the window of opportunity is perilously small and the market is unforgiving if errors are not immediately corrected. Using KPIs lets companies prepare better for a successful launch from both external and internal perspectives (Figure 1).
External benefits
Externally, the success of any given launch is driven by its use in the market as it progresses through the cascade from advocacy, approval and market access to adoption and, ultimately, adherence. To plan for a successful launch, marketers must address five steps in their preparation:
1. Achieving brand advocacy among regulators, payers, key opinion leaders (KOLs), prescribers, patients and other stakeholders;
2. Gaining brand approval with optimal labeling for the right patients;
3. Securing market access on the most favorable terms with national and local payers, health systems and medical groups;
4. Attaining brand adoption for the optimal patient segments with a focus on working with prescribers and providers to achieve early, strong positioning in the dynamic market (new, switch, and, if relevant, add-on patients); and
5. Ensuring brand adherence by retaining patients as loyal repeaters for as long as is clinically appropriate.
KPIs track progress towards these external goals by measuring progress towards achieving relevant access with formularies, gaining physician adoption, and understanding whether prescribers try out the product and convert into regular users.
Internal benefits
From an organizational perspective, KPIs can offer powerful insights into the status of internal preparations. They enable consistent tracking of alignment on objectives, strategy, definition of success and critical success factors, as well as visibility into launch plans and performance, and overall quality and timeliness.
Identifying the right KPIs is a key first step in preparing for launch success. Simply establishing a set of KPIs is not sufficient, however. The second key step is ensuring that they can be tracked to target so that gaps can be made visible, interpreted appropriately and acted on quickly should any of the metrics fail to reach a satisfactory value. As part of such a structured process, KPIs are pivotal to achieving the brand vision and business objectives by helping launch teams gain near-immediate awareness of issues to which they can rapidly respond. This is particularly relevant given the vital importance of the first few weeks post-launch, when the long-term growth trajectory of most new brands is established.
CHALLENGES TO IMPLEMENTING KPIs
While designing and implementing a KPI framework may seem intuitive, many companies flounder in the process. Failure to use a holistic set of metrics and execute well on those metrics are two common issues that impede their optimal use.
Failure to use holistic metrics. First, in launch situations, many pharmaceutical companies still track brand performance using sales, share and growth. While important, these conventional metrics are essentially lagging indicators and are of minimal value in providing the granular information needed to ensure an optimal course. Moreover, they cannot be used pre-launch.
The goal is to advance this conventional perspective to include relevant, insightful and actionable KPIs (Figure 2). These should be a combination of leading and lagging indicators that are specific to the brand and in line with its critical success factors. They should allow for tracking of both launch preparation and early success in the market, measuring progress towards organizational alignment, the development of a pertinent value proposition and effective stakeholder engagement.
Poor execution. A second key challenge lies in the design and implementation of an optimal KPI solution. Here, marketers frequently encounter a number of pitfalls (Figure 3).
In many cases, for example, companies continue to use analogues that are 10-15 years old when more recent benchmarks reflecting changes in the launch environment would be appropriate. Another major problem is including too many KPIs, a clear indication that most of them are probably not “key” at all. Imbalance between KPIs across functions and regions and failure to link KPIs to critical success factors are also widespread. Furthermore, the selected KPIs may be relevant globally, not tailored to local need, as aggregation on a global level is important for senior management reporting. At the same time, though, KPIs must also provide an opportunity to drill down at the local level. And, critically, the omission of a governance process leaves many companies unable to react in good time when remedial action is required.
As a consequence, investment in KPI systems is often wasted. Many fail to contribute directly to performance; others are simply useless; some can even be counter-productive.
DEVELOPING A BEST-IN-CLASS SYSTEM
The goal of an effective KPI system is the ability to track and benchmark performance regularly and consistently across functions and markets, using metrics which provide early indicators of progress that are linked to corrective actions. A best-in-class framework should thus consist of insightful and actionable metrics, clear definitions and benchmarks, a reporting process that requires minimal manual intervention, and governance oversight that enables fast, proactive responses. All parties must agree on the chosen KPIs, benchmarks and targets and be clear on how action will be triggered from the management system.
There are four essential steps to achieving the optimal structure:
Define the metrics
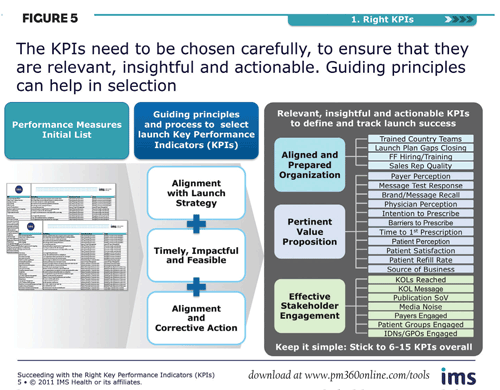
Well-defined and monitored KPIs are central to steering an optimal launch course. Choosing the right ones is essential. They should be: relevant to the therapy area, product strategy and geography; clearly linked to critical success factors; focused on market results or output quality; and no more than 6-15 in total number (Figure 4).
As a guiding principle, KPIs can be designed against the three dimensions of launch success:
- An aligned and prepared organization: These KPIs focus on ensuring internal preparation, and should ensure that adequate resources are available, country and functional teams are fully up to speed on the launch, clinical and commercial teams are accountable for the quality of delivery, and plans are in place for closing gaps.
- Pertinent value proposition: Here, the initial focus is on ensuring that the value proposition is powerful, relevant, and accepted by all key stakeholders—including payers, physicians and patients. This can be measured by KPIs that address such issues as message response, brand/message recall, intention to prescribe, physician perception, etc.
- Effective stakeholder engagement: KPIs tracking brand impact in the market can include the number of KOLs reached, publication share of voice, degree of noise in the media, level of patient groups engaged, etc.
It is important to ensure that the chosen KPIs are aligned with the overall launch strategy, concentrating only on those that will make a difference. They should be a mix of pre- and post-launch KPIs, as well as measures that are launch-independent —these last focus on such areas as disease awareness and education, scientific publications, and KOL contacts.
As a guide, some key questions to consider when establishing launch KPIs include:
- Are the KPIs aligned to the launch strategy and critical success factors for the brand?
- Is there a good mix of leading and lagging indicators across the three dimensions?
- How will performance be tracked against the metric, and what are the expectations?
- How quickly can a performance gap be detected and corrective action be taken?
- Who will take the action—and when?
Establish definitions and benchmarks Prior to implementation, each KPI should be defined in detail, to ensure clarity on metrics and feasibility. In tracking KOL engagement, for example, it is important to establish exactly what this means. Does it imply that KOLs are speaking well of the brand or actively advocating its use? What is the hierarchy in terms of reaching national and regional KOLs? How will engagement be measured? And who is responsible for monitoring and reacting if remedial action is necessary? Benchmarks are critical to ensuring alignment on the chosen targets and should be set as soon as the final KPI framework has been approved. They can be gathered using analogue selection either in partnership with an external vendor or derived from secondary data, experts, stakeholder input, and the launch goals themselves. Leverage multiple available data sources to address the key needs of the tracking system, including sell-in, sell-out data, chart audits and longitudinal patient information. In the interests of ensuring a cost-effective KPI system, these should all be considered ahead of recourse to primary research.
Enable visibility
Providing all stakeholders with a clear view of launch preparations and performance across functions and geographies is essential. An easy-touse tracking dashboard provides an effective tool for creating transparency across the entire organization and ensuring the availability of up-to-date information on each of the chosen parameters and metrics.
Incorporate a governance process
A strong, customized governance structure, consisting of a crossfunctional and geographical team and a process for escalation and resolution is key to ensuring alignment on the performance tracking system, as well as providing clarity on how action will be triggered. Critically, it positions companies with maximum lead time in ensuring the launch is on track, with the ability to make rapid adjustments as required. (Figure 5)
A FOUNDATION FOR EXCELLENCE
The imperative to maximize product uptake in the narrowest window of time places heavy demands on every new launch in today’s risky and challenging environment. The elements that secure a successful trajectory are complex and product-specific. The launch window is short and unforgiving. Errors are costly if not immediately corrected. Going forward, brands that excel at launch will be supported by companies that prioritize the planning and monitoring process, using a best-practice KPI framework specifically designed for their product. The pitfalls are many and the challenges are growing—but by following a transparent, consistent approach and choosing the right KPIs for their brand, companies can achieve an effective and accessible reporting structure that is well understood, includes benchmarks derived from custom-selected analogues, combines various data sources in an up-to-date tracking tool, delivers regular reports in a timely manner, and supports the planning of appropriate actions to keep launch performance on track.
FIGURE 1:
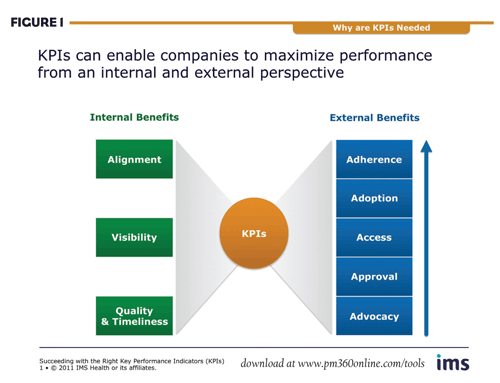
FIGURE 2:
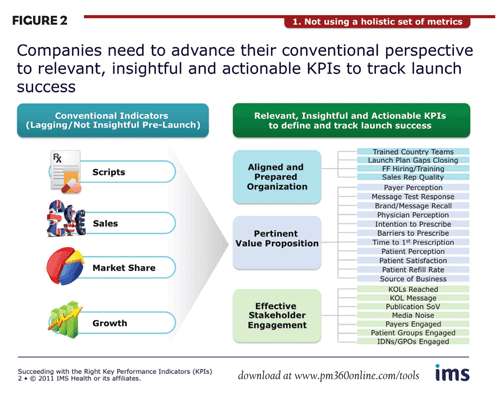
FIGURE 3:
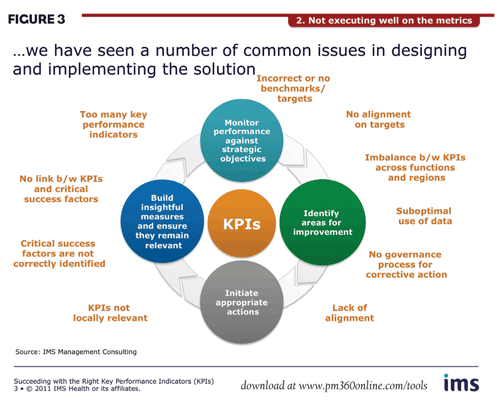
FIGURE 4:
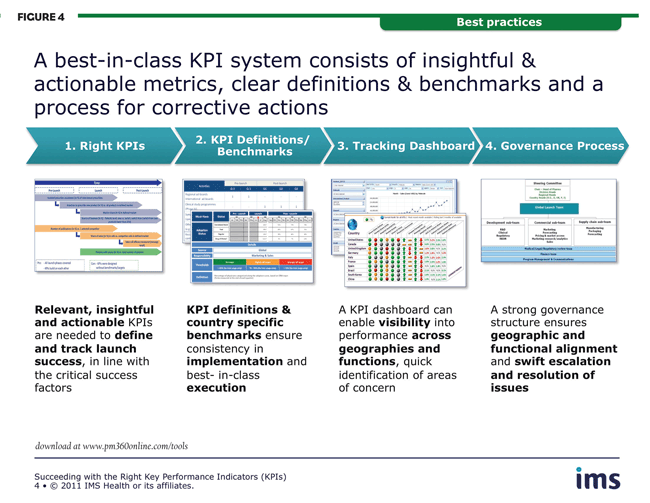
FIGURE 5:
REFERENCES
1. IMS definition as described in Pharmerging Shake-up, IMS White Paper, March 2010 2. IMS Launch Excellence studies of 4000 launches in over 90 therapy areas across eight different countries over 10 years






