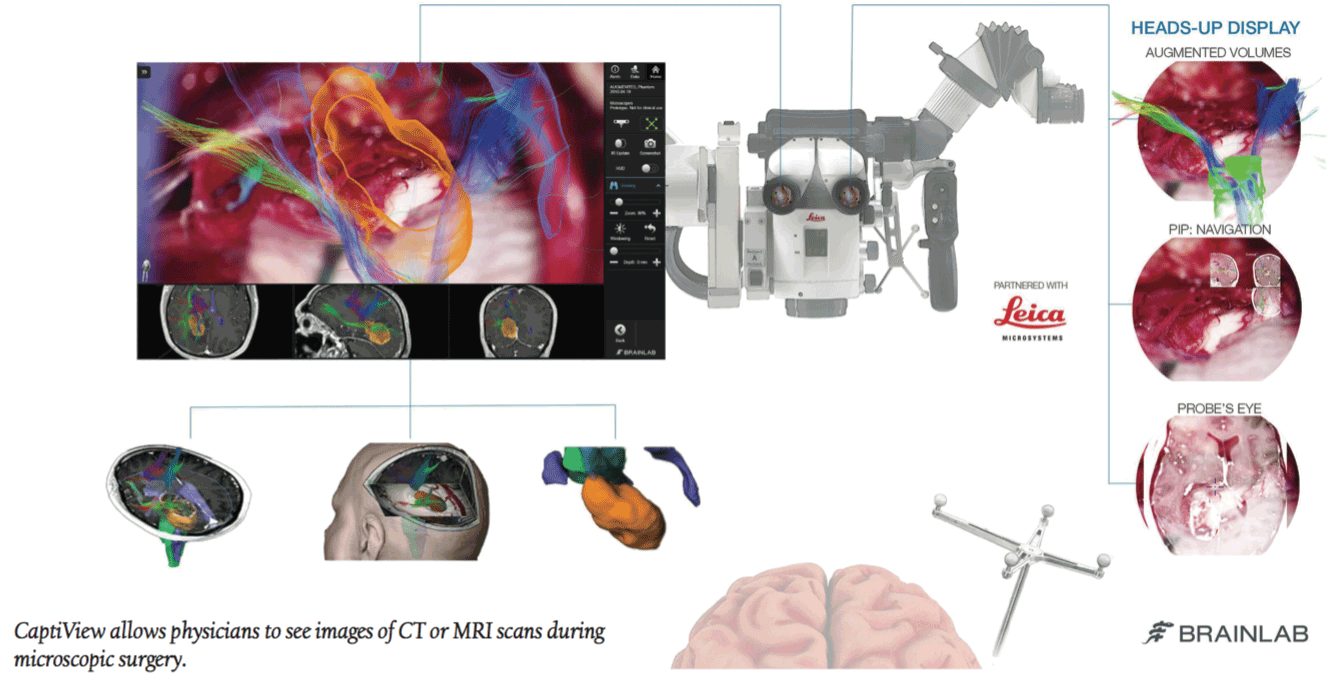
Medical Devices: Augmented Reality in the OR
Leica Microsystems is using the augmented reality technology typically seen in video games in their neurosurgery equipment. Dr. Joshua Bederson, System Chair for the Department of Neurosurgery at Mount Sinai Health, became the first neurosurgeon to use CaptiView, a microscope image injection system that overlays critical virtual reality imaging directly onto the brain when looking through the eyepiece.
Doctors using Captiview can see images of CT scans or MRIs during microscopic surgery. Hand knobs and foot switches allow surgeons to flip thru live imagery and other images without interrupting the surgery. “We are driving and advancing the development of next-generation simulation and virtual reality technology, which can help improve patient outcomes and solve neurosurgical challenges,” says Dr. Bederson. He now uses CaptiView for all his neurosurgical operations and works with Leica to improve the technology.
TeleMed Text: Wearables 2.0
Engineers from Tufts University have found a way to integrate nanosensors into cotton and synthetic threads, to penetrate multiple tissues and gather real-time diagnostic data. The threaded sensors were tested in rats and successfully reported tissue health, pH and glucose levels, how well a wound is healing, and signs of infection. “The ability to suture a thread-based diagnostic device intimately in a tissue or organ environment in three dimensions adds a unique feature that is not available with other flexible diagnostic platforms,” says Sameer Sonkusale, PhD, Director of the Nano Lab in the Department of Electrical and Computer Engineering at Tufts School of Engineering.
“We think thread-based devices could potentially be used as smart sutures for surgical implants, smart bandages to monitor wound healing, or integrated with textile or fabric as personalized health monitors and point-of-care diagnostics.” The materials for this sensory equipment are inexpensive, thin, and flexible, making them the perfect new options for convenient wearables.
Discoveries/Innovations: Replacing Hip Replacements
Whether from tougher sports requirements or genetics, hip arthritis is becoming more common for younger generations. While hip replacement has been a great solution for deteriorated hip joints, this surgery is not ideal for youngsters because prosthetics last less than 20 years. Replacing prosthetics requires a difficult surgery that could cause more permanent bone damage and infection. Ali Ross, a PhD student at Washington University and her mentor Farshid Guilak, PhD have developed an alternative prosthetic formed from stem cells.
Scientists have already shown that they can make stem cells grow on a 3D scaffold shaped like a hip joint, as well as use gene therapy to make these stem cells release anti-inflammatory molecules that can prevent future arthritis. The scaffold is molded to the exact shape of the patient’s joint and will biodegrade once surgically inserted. It is covered in cartilage with stem cells that will then develop into a new joint, hopefully eliminating the need for a total hip replacement in the future. Along with the gene therapy used to stave off inflammation, scientists hope this will keep arthritis from returning entirely.
Study Sessions: We Have A Lot To Learn About Biosimilars
Biosimilars have become the most promising treatments for U.S. patients who can’t afford expensive biologics. Researchers at Johns Hopkins University and Brigham and Women’s Hospital conducted a study on anti-inflammatory biosimilars to determine how they compare with brand-name drugs in safety and efficacy. They’ve concluded that biosimilars are interchangeable with biologic therapies—but many scientists and doctors are concerned that the research hasn’t revealed anything new—and the lack of available biosimilars to study don’t offer very robust results. So far, the molecules in biosimilar drugs seem almost identical and haven’t caused adverse reactions. However, it seems likely that insurance companies will push patients towards biosimilars to save billions, while only a very limited amount of knowledge on their effects is available.
Therapeutic Talk: New Genomic Info on Depression
A study has finally succeeded in shedding some light on the huge role genetics play in depression, a debilitating condition that affects more than 350 million individuals worldwide. Pfizer and Massachusetts General Hospital evaluated huge amounts of data pulled from surveys taken by 23andMe customers. It revealed that 17 single nucleotide polymorphisms in 15 genetic loci are major influencers of depression in Europeans.
Because depressions run a spectrum and genes and environmental factors influence its development, only a very large set of data could yield any conclusive signals. 450,000 23andMe customers, 120,000 of whom are diagnosed with depression, contributed to this data, which allowed scientists to see that the gene region involved with brain function has the strongest association with the condition. MEF2C and TMEM161B have the strongest association. MEF2C is involved in the regulation of synapses in the brain. Most of the other 13 significant genes are expressed in the central nervous system. Researchers hope these findings will shed light on the biology of the disease and help find effective treatments.
FDA Update
Drug Approvals
Sustol, the first extended release 5-HT3 receptor antagonist manufactured by Heron Therapeutics, received priority review approval for its ability to prevent delayed nausea and vomiting associated with moderately emetogenic chemotherapy and anthracycline and cyclophosphamide combination regimens. It prevents both acute and delayed nausea and vomiting.
Breakthrough Therapy
Clovis Oncology’s rucaparib, a PARP inhibitor, received breakthrough therapy designation as a monotherapy treatment of advanced cancer in patients who have undergone two prior lines of platinum-containing therapy. It is indicated for use in those with BRCA-mutated tumors, inclusive of both germline (gBRCA) and somatic (sBRCA) mutations.
Orphan Drug Designation
The FDA recently granted Regenicin orphan drug designation for its NovaDerm cultured skin substitute. According to the company, this is the only autologous cultured skin substitute prepared using the patient’s own skin cells. The product is a biologic indicated for treatment of patients with thermal burns on greater than 30% of the body.
Biotie’s BTT1023 drug candidate for the treatment of primary sclerosing cholangitis (PSC) recently received orphan drug designation from the FDA. PSC is a chronic, progressive disease, and as of now, the FDA has approved no treatments for this illness.
Med Device Approval
The first intraocular lens designed to extend the depth of focus for cataract patients, the Tecnis Symfony IOL, manufactured by Abbott Medical Optics, is approved for extending the depth of focus and increasing the sharpness of vision for cataract patients who otherwise have clouded vision. The new technology may reduce the need to wear contact lenses or glasses after cataract surgery.