Trend Setting: Marfan Foundations’ 2018 Heroes with Heart Make a Difference

The Marfan Foundation’s 2018 HeartWorks Gala, an annual event supporting the foundation’s life-saving programs for Marfan Syndrome patients, will honor Gil Bashe, Managing Partner, Global Health, Finn Partners with a Hero with Heart Award. Bashe, a long-time Marfan Foundation supporter and volunteer at the American Heart Association for 26 years, will be honored as a powerful health influencer who uses communication to improve quality patient care. Bashe says, “Finn is all about making a difference in the world and I am humbled that our mission enables me to be a counselor to clients as well as an advocate for improved patient care.”
Alongside Bashe, Ann Reinking, a Tony Award-winning actor, dancer, and choreographer, and Dr. Reed E. Pyeritz, a medical geneticist at Perelman School of Medicine at the University of Pennsylvania, will also be named Heroes with Heart. Reinking’s son Chris, who is diagnosed with Marfan syndrome, inspired her award-winning documentary In My Hands, and her passionate support of Marfan research. Aside from serving as a valuable Marfan researcher, Dr. Pyeritz is a founder of the Marfan Foundation and dedicated advisor.
Bashe uses his extensive communication expertise to open access to life-saving information about the lesser known, fatal disorder. “We are honored to present Gil with a Hero with Heart Award for his dedication to heart health, including conditions like Marfan syndrome, which, without prompt diagnosis, cut lives short,” says Michael Weamer, President and CEO of The Marfan Foundation, in a statement. “He is an advocate for ensuring patients and their families have access to information and health and supporter of our organization.”
Marfan syndrome is a life-threatening genetic disorder of the body’s connective tissue. It affects the heart and blood vessels, the bones, and the eyes. The HeartWorks Gala, held on May 22nd, 2018 in New York City, has raised more than $13 million for life-saving and life-enhancing programs and services for the 200,000 people in the U.S. living with Marfan syndrome and related disorders.
Doctor Docs: Outcome Health Partners with ACC For Heart Health
Outcome Health and the American College of Cardiology have partnered to take on the ambitious goal of improving heart health across the U.S. by helping doctors deliver the information patients need via digital devices. Together, through this new patient empowerment and education initiative, CardioSmart, the companies will collaborate to develop technologies that patients can use to live a heart-healthy lifestyle. Outcome’s already existing Exam Room Tablet onboards patients to lifestyle management programs and provides interactive resources and health assessments pre-consultation. The company’s offerings also include the Digital Wallboard, which renders 3D images of the heart for physicians to use to better communicate with their patients during consultation.
“The American College of Cardiology takes a holistic approach towards transforming cardiovascular care and heart health,” Anil Harjani, Vice President of Product Management and Strategic Partnerships at Outcome Health, said in a statement. “We look forward to adding another dimension to this important work through our platform. Together, we strive to deliver the best outcome possible to the millions of patients, physicians, and caregivers that we impact.”
TeleMed Texts: Apple Sets Sights on EHR Industry
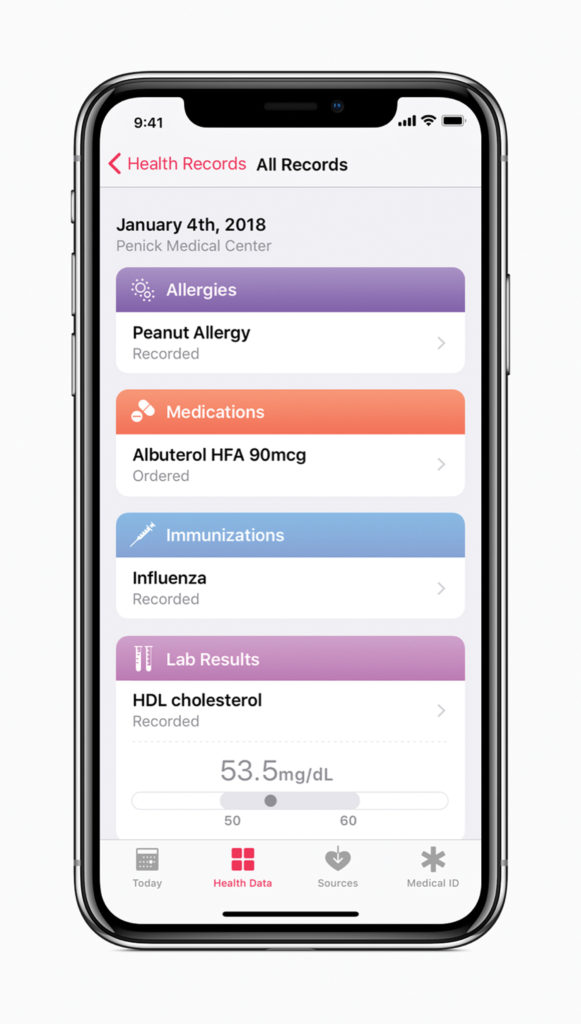 Apple has a reputation for majorly disrupting every industry it enters (computer, music, phone, just to name a few). Now the influencer is on a mission to democratize the EHR industry, and this has some far-reaching implications for pharma. Apple has already put power into the patient’s hand by allowing anyone to aggregate and view their own medical records from hospitals, labs, clinics, and more—all on their Health app. This doesn’t only have the potential to increase patient engagement with their own care, but can help them engage with their providers, and perhaps pharma.
Apple has a reputation for majorly disrupting every industry it enters (computer, music, phone, just to name a few). Now the influencer is on a mission to democratize the EHR industry, and this has some far-reaching implications for pharma. Apple has already put power into the patient’s hand by allowing anyone to aggregate and view their own medical records from hospitals, labs, clinics, and more—all on their Health app. This doesn’t only have the potential to increase patient engagement with their own care, but can help them engage with their providers, and perhaps pharma.
Ideally, patients’ healthcare visit records will automatically be uploaded to their Health app and they have the instant ability to share these documents with whomever they choose. As patients become more excited about self-care, they may want more direct information from the pharma market—a golden opportunity for pharma marketers to share tailored content based on their digital health data.
“In much the same way that Apple suggests music to you through iTunes, they could be suggesting health interventions to you through your Health app. Where you wouldn’t be sharing your data with the broader world, you’d just be showing an anonymized set of your data so that a third party can provide an anonymized recommendation,” says Destry Sulkes, MD, Chief Data Officer at WPP Health & Wellness.
Somewhere down the road, pharma could strike a deal with Apple, using their customer’s health data to personalize user experiences, apps, ads, or even direct interaction.
Discoveries/Innovations: Boston Children’s Hospital and Klick Bring Virtual Reality to Pediatric Patients
The HealthVoyager is a (supercool) medical education and patient experience platform—a Proof of Concept that uses Virtual Reality (VR) Technology to bring a patient’s individual medical findings to life in an immersive 3D experience. The tool is designed for pediatric gastrointestinal (GI) patients.
Kids at Boston Children’s Hospital—along with their parents—can now experience a tour of their own gastrointestinal tract that shows conditions such as Crohn’s Disease or ulcerative colitis so that the kids know exactly what is happening inside them through engaging, fun, and modern technology. The HealthVoyager is currently under clinical study to validate its effect on patient and family understanding, engagement, and satisfaction.
“Putting myself in a nine-year-old’s shoes, I can see how HealthVoyager could be a much more fun and valuable way to learn about and share complicated information like endoscopic findings,” Michael Docktor, MD, a pediatric gastroenterologist and Clinical Director at BCH who co-developed the tool, said in a statement. “We hypothesize that the more children and their families can visualize and understand their disease, the more likely they may be to communicate when they have a particular symptom and adhere to their therapies.”
Med Device: First Smart Watch to Detect Seizures Gets the Green Light
 Empatica Inc will soon be marketing Embrace, the first-ever smart watch designed to recognize seizures and manage epilepsy. Machine learning allows the medical device to identify convulsive seizures by subtle electrical changes that occur on the surface of the skin and then send instant notifications via text and an automated phone call to assigned caregivers. Embrace is equipped with a sympathetic nervous system sensor, a gyroscope, a 3-axis accelerometer to detect high sensitivity motion, and a peripheral skin temperature sensor to provide the most accurate information to a smartphone app.
Empatica Inc will soon be marketing Embrace, the first-ever smart watch designed to recognize seizures and manage epilepsy. Machine learning allows the medical device to identify convulsive seizures by subtle electrical changes that occur on the surface of the skin and then send instant notifications via text and an automated phone call to assigned caregivers. Embrace is equipped with a sympathetic nervous system sensor, a gyroscope, a 3-axis accelerometer to detect high sensitivity motion, and a peripheral skin temperature sensor to provide the most accurate information to a smartphone app.
FDA Update
Drug Approvals
Eli Lilly and Company received additional FDA approval of Verzenio (abemaciclib) as an initial endocrine-based therapy for postmenopausal women with advanced or metastatic breast cancer when used in combination with an aromatase inhibitor (AI).
Imfinzi, AstraZeneca’s newly launched lung cancer therapy, received full FDA approval. The intravenous drug can be used by patients with unresectable Stage III non-small cell lung cancer whose disease has not progressed following chemotherapy and radiation therapy.
Janssen Pharmaceuticals also received FDA approval for a cancer drug called Erleada for the treatment of prostate cancer that has not spread, but continues to grow despite treatment with hormone therapy (castration-resistant). The first drug to treat this condition, Erleada works by blocking the effect of androgens, which are hormones like testosterone, on the tumor.
Priority Review
The FDA has accepted Tetraphase Pharmaceuticals’ drug eravacycline for a six-month priority review that should be completed in August. The antibiotic is intended for the treatment of complicated intra-abdominal infections and other infections caused by multidrug-resistant pathogens.
Med Device Approvals
The FDA has approved Hologic Inc.’s Aptima HBV Quant Assay, a laboratory test that measures the amount of Hepatitis B Virus in a patient’s blood. After the test is conducted in a lab using Hologic’s Panther system, a physician can use the results to determine how well the virus is responding to treatment and help them guide patients undergoing antiviral therapy for chronic HBV infection.
Abiomed Inc. received expanded FDA approval for its Impella 2.5, Impella CP, Impella 5.0, and Impella LD heart pumps. The new indication now includes treatment for heart failure associated with cardiomyopathy leading to cardiogenic shock.