Therapeutic Talk: Janssen Tackles the Depression Medication Gap

While antidepressants have helped many patients manage depressive symptoms, about a third of sufferers are still searching for an effective treatment. Scientists at Janssen are exploring a new therapy known as glutamine receptor modulators that seem to be the answer for restoring synaptic connections in brain cells of those with treatment-resistant depression. Esketamine, Janssen’s newest exploratory medicine for major depressive disorder, has the exciting potential to improve the lives of these 100 million globally.
Phase 2 clinical results recently demonstrated a significant, clinically meaningful, rapid improvement of depressive symptoms as compared to intranasal placebo. Husseini K. Manji, MD, Global Head, Neuroscience Therapeutic Area at Janssen, said in a statement, “The results of this study reinforce the potential of esketamine as a treatment for patients with treatment-resistant depression and support further clinical research, providing hope for people in need. If approved by the FDA, esketamine would be one of the first new approaches to treat refractory major depressive disorder available to patients in the last 50 years.”
Medical Device: A New Way To Manage IBS
 Biomerica, a global biomedical company, has launched a product that will redefine how patients treat and manage IBS symptoms. The InFoods IBS diagnostic therapy tool allows the patient to take a simple blood test that can identify specific foods that trigger IBS symptoms in just 15 minutes.
Biomerica, a global biomedical company, has launched a product that will redefine how patients treat and manage IBS symptoms. The InFoods IBS diagnostic therapy tool allows the patient to take a simple blood test that can identify specific foods that trigger IBS symptoms in just 15 minutes.
Unlike traditional medications, the tool can be used by patients of all three IBS types and has zero side effects. The tool helps patients take control of their IBS by recognizing the foods that affect the individual, rather than trying to avoid a whole list of foods that might trigger symptoms in the entire population of IBS sufferers.
Discoveries/Innovations: Researchers Make the Most Important Discovery in Cardiology in Years
Researchers accidently stumbled upon a previously unheard of phenomenon occurring in the white blood cells of bone marrow that is clearly linked to heart attacks and strokes. Cardiologists have always known and wondered why such a large population of patients who have normal cholesterol, don’t drink or smoke, don’t have a family history of heart issues, or any other conventional risk factors, are still victims of cardiovascular disease. Now they may have an answer.
Several research teams have discovered that 20% of people in their 60s and at least 50% of people in their 80s acquire a white blood cell mutation known as CHIP. Using databases from genetic studies involving tens of thousands of people, it was proven that white blood cells will eventually acquire this clonal hematopoiesis of indeterminate potential (CHIP) for no clear-cut reason. That increases a person’s risk of dying within a decade—usually from a heart attack or stroke, by 40% or 50%.
Our white blood cells, which originate in bone marrow, divide rapidly into billions of new cells. By chance, some cells acquire a mutation that causes them to accumulate in the marrow, passing it on to billions of offspring. While this type of mutation would normally point to leukemia, scientists found that a large majority of patients displaying CHIP had no signs of cancer, but instead have had fatal heart attacks or strokes.
White Blood Cells Incite Inflammation
“For decades people have worked on inflammation as a cause of atherosclerosis,” said Dr. Benjamin Ebert, Chair of Medical Oncology at the Dana-Farber Cancer Institute. “But it was not clear what initiated the inflammation.” The plaque responsible for obstructing many people’s arteries are filled with white blood cells that incite inflammation, offering cardiologists the crucial answer to how fatal inflammation begins in our arteries and why CHIP is so closely linked to heart disease.
For now, CHIP seems to be an unavoidable part of aging, but this makes it all the more important to control other heart disease risks by maintaining cholesterol and blood pressure.
TeleMed Texts: Facebook is Finally Ready for Pharma
 Facebook holds plenty of summits regarding advertising and partnerships with an array of industries. But last year, drug marketers had their first sit down with the giant social network. The summit not only focused on mobile marketing and setting advertising budget standards for pharma and OTC manufacturers, but also on convincing drug makers of the safety and effectiveness of social media marketing.
Facebook holds plenty of summits regarding advertising and partnerships with an array of industries. But last year, drug marketers had their first sit down with the giant social network. The summit not only focused on mobile marketing and setting advertising budget standards for pharma and OTC manufacturers, but also on convincing drug makers of the safety and effectiveness of social media marketing.
Opening to Branded Promotions
It seems Facebook would like to move away from disease-awareness campaigns and bring in more branded promotions. “We’ve seen the indicators now that people want more of this, and hopefully we’ll be able to make it even bigger next year and extend invites even further,” comments Meredith Guerriero, Facebook’s Director of the Health, Grocery, Drug and Politics verticals. “It’s also just about bringing the industry together. There are not many forums where they can all come together—and live and breathe within the Facebook environment as well.”
The popular summit will now be held annually, and Facebook hopes to host other events to educate and brainstorm with industry experts to become pharma marketer’s social media go-to.
Facebook has been making major changes to meet the demands of the industry, such as incorporating the ability to track comments made by the consumer community in compliance with pharma’s patient feedback responsibilities. The social media giant has also relaxed some of its text policies to allow FDA-mandated disclosures for brand-name drugs to accompany an ad. Facebook’s efforts seem to be ushering in a new age of social media campaigning for pharma marketers across the industry.
DC Dispatch: Children’s Healthcare is Safe—For Now
After months of debate, Congress reauthorized the Children’s Health Insurance Program (CHIP) for six months, passing within the bill that ended the three-day government shutdown. States have been using money reserves to keep their programs going, but at least nine million low-income children can now receive healthcare worry free for a few more months.
Come July, Republicans and Democrats will have to come to an agreement on how to pay for the program to keep it in place. Already, a Senate bill is in the works that would extend CHIP for five years while Congress negotiates restoring the funding for the program. Democratic U.S. Senator Doug Jones, Alabama’s state Senator, is the latest of 24 Senate sponsors of the Keep Kids’ Insurance Dependable and Secure (KIDS) Act of 2017.
“I’m proud to announce that the first piece of legislation I’ll co-sponsor will ensure a long-term funding solution for the Children’s Health Insurance Program,” Jones said in a statement. “I have said from day one that my first priority—when I arrived in Washington—would be to protect the 150,000 Alabama children, and the nine million more across the nation who depend on CHIP for their healthcare. This bipartisan plan would extend CHIP funding until FY 2022, which will ensure low-income families can continue to count on this critical program—and it will protect states from having to foot the entire bill. It is long past time to renew CHIP and I’m proud to join my colleagues who are fighting for the children who depend on it.”
Trend Setting: Once-a-Week HIV Treatment Opens an Adherence Avenue
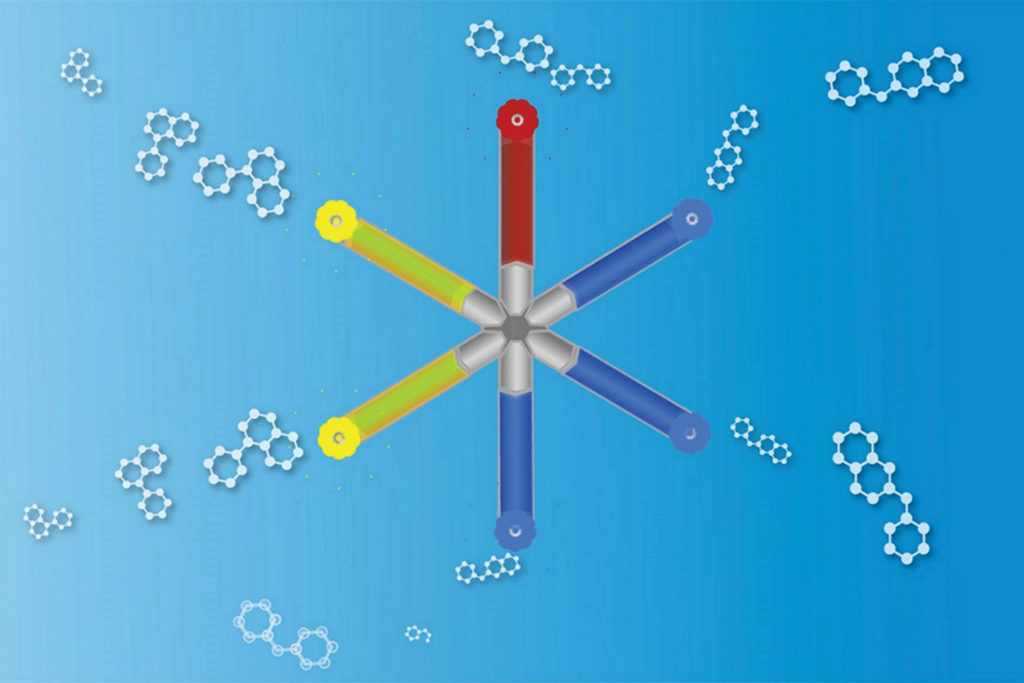 Researchers at MIT and Brigham and Women’s Hospital have found that replacing daily pills with a weekly medicine could help HIV patients adhere to their medicines, so they’ve developed a weekly therapy to make it happen. A specially designed capsule holds a week’s dosage of HIV drugs and releases them gradually, making it easier to manage the strict regimen needed to fight the aggressive virus. The system could even be used by those at risk of HIV exposure to help prevent infection.
Researchers at MIT and Brigham and Women’s Hospital have found that replacing daily pills with a weekly medicine could help HIV patients adhere to their medicines, so they’ve developed a weekly therapy to make it happen. A specially designed capsule holds a week’s dosage of HIV drugs and releases them gradually, making it easier to manage the strict regimen needed to fight the aggressive virus. The system could even be used by those at risk of HIV exposure to help prevent infection.
Because of the array of different drugs needed for treatment, one major obstacle in testing and measuring the success of antiretroviral drugs to prevent HIV infection in healthy populations has been getting people to take all the necessary pills every day. The capsule is constructed in a star shape so that each of the size arms can be loaded with a different drug polymer. The capsule can be customized to release each drug at varying rates. As the research moves into clinical trial phase, Robert Langer, the David H. Koch Institute Professor at MIT and a senior author of the study says, “We are all very excited about how this new drug-delivery system can potentially help patients with HIV/AIDS, as well as many other diseases.”
While the life sciences industry has long been concerned with adherence, the trend has been to develop new digital apps and devices to keep patients on track with their medical maintenance. These researchers’ new approach to adherence may help patients adhere to dosage schedules even more by limiting the amount and frequency of drugs taken altogether.
FDA Update
Drug Approvals
Shire earned the FDA’s Breakthrough Therapy designation for its drug maribavir, which is currently demonstrating considerable success in Phase III studies. Maribavir is an investigational treatment for cytomegalovirus infection and disease, a beta herpes virus that causes problems that can be fatal in transplant patients resistant to prior therapies. With Breakthrough Therapy designation, the FDA provides intensive guidance on an efficient drug development program and priority review of the marketing application.
Novartis drug Promacta also received FDA Breakthrough Therapy designation. The drug is intended as a first-line therapy for patients with severe aplastic anemia (SAA), a rare blood disorder in which bone marrow fails to produce enough red blood cells, white blood cells, and platelets. It is already approved as a second-line therapy for SAA, as well as for patients with chronic immune thrombocytopenia.
Med Device Update
The PreveLeak Surgical Sealant received an expanded device indication to help stop leaks formed in tissue or blood vessel during circulatory system surgeries. Mallinckrodt Pharmaceuticals received the FDA’s go ahead to market the product in the U.S.





