Abbott’s COVID Test Available Now
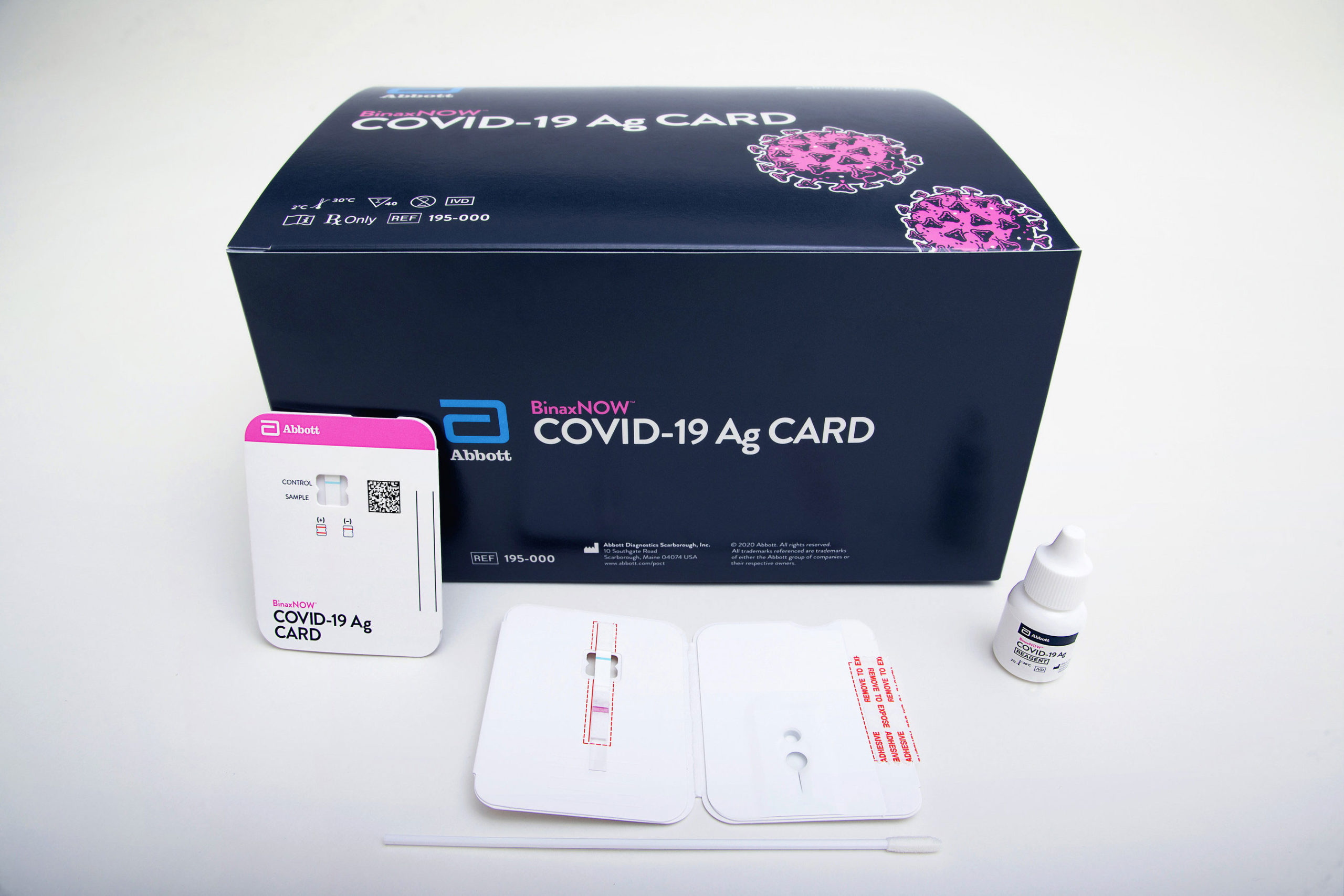
The FDA has approved Abbott’s prescription COVID-19 diagnostic test, BinaxNOW, which will be sold for $5 and is about as big as a credit card. The company developed Navica, a free smartphone app, that will work with the rapid antigen test and store the reading so it can be used as a “temporary health pass” for those who need test results to enter establishments like hospitals and schools. Results from the nasal swab reading are ready in 15 minutes and sent automatically to the app. The test will be available with a prescription and performed by healthcare professionals, including school nurses, medical assistants, pharmacists, and employer occupational health specialists.

“This new COVID-19 antigen test is an important addition to available tests because the results can be read in minutes, right off the testing card. This means people will know if they have the virus in almost real-time,” stated FDA Device Center Director, Jeff Shuren. “Due to its simpler design and the large number of tests the company anticipates making in the coming months, this new antigen test is an important advancement in our fight against the pandemic.”
Abbott will be shipping more than 10 million tests this month and ramping up production to 50 million in October. Clinical studies found the test to have a false-positive rate of 1.5% and a false-negative rate of 2.9%. The company says it is best used within seven days of symptoms appearing to identify those who should isolate.
FDA Eager to Review a Vaccine Before Presidential Election
FDA Commissioner Stephen Hahn, MD announced on Twitter that the agency looks forward to reviewing Pfizer’s coronavirus vaccine on October 22nd, days before the U.S. election. Pfizer is in the middle of Phase 3 clinical trials and expects to have enough data for review by the FDA meeting deadline. Pfizer has made it clear they intend to seek FDA approval in October, and Dr. Hahn’s comments confirm that with enough scientific evidence, they will grant FDA Emergency Use Authorization to the company before November.
Through its partnership with BioNTech, Pfizer developed the BNT162b2 mRNA which produced confirmed responses in CD4+ and CD8+ T cells in human trials. Experts say this may be a strong indicator for an immune response and differentiates the vaccine from Moderna’s, the other mRNA vaccine candidate. In the U.K., AstraZeneca is in Phase 2 of clinical trials with efficacy results expected later this year, while Sanofi and GlaxoSmithKline are entering Phase 3 trials for their protein subunit vaccine.
Moving Remdesivir Outside the Hospital
Gilead’s injectable remdesivir is still the only authorized COVID-19 therapy in the U.S., given to patients hospitalized with the virus. Researchers at the University of Texas are developing a powdered version of the drug for inhalation, which would be more accessible to those out of the hospital and suffering from milder cases of the coronavirus. Using thin film freezing technology, the researchers are turning remdesivir into a powder that they then load into an inhaler, delivering a more affordable, lower dose of the medication directly into the lungs.
“The rapid freezing technology turns drugs that don’t easily dissolve in water into a ‘brittle matrix powder,’ a fluffy powder that’s ideal for pulmonary administration,” Robert O. Williams III, PhD, researcher at the school’s Division of Molecular Pharmaceutics and Drug Delivery, said in an interview with FiercePharma. “The final product is amorphous, not crystalized, which means it aerosolizes and dissolves easily in the lungs.”
Williams hopes to pitch the formulation to Gilead, who needs to work out a deal before this therapy could hit the market. It may be a hard sell considering the company is testing its own inhalable, nebulized remdesivir solution for the same purposes.