ZimVie’s Mobi-C Expanded Implant Offering and Hybrid Study
Advancing the Care of Degenerative Cervical Spinal Disease
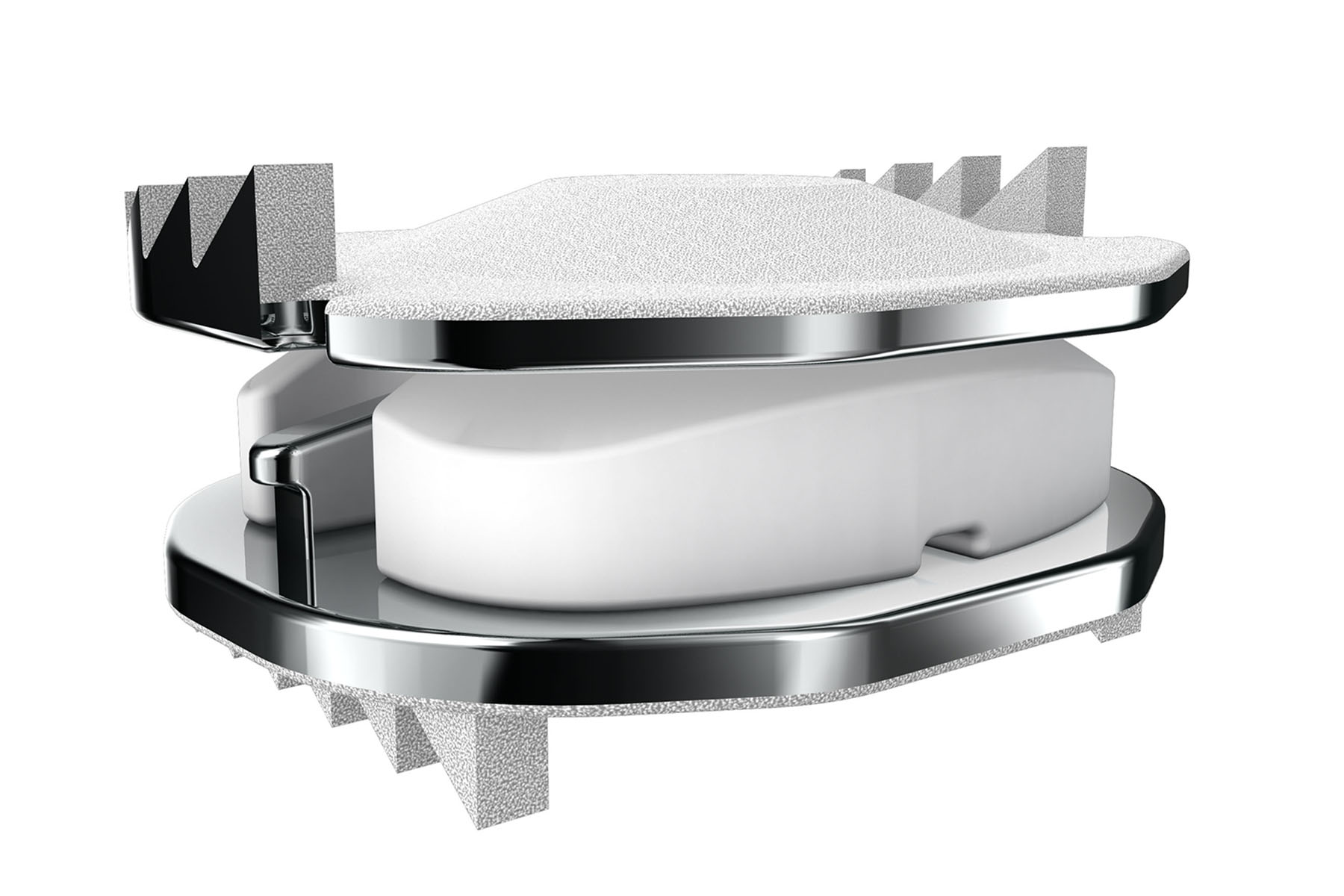
Degenerative cervical spinal disease is a condition affecting the neck’s spinal discs, causing pain and discomfort. Restoring natural motion to the cervical spine with disc arthroplasty (artificial disc replacement) has become an emerging standard of care. ZimVie introduced the Mobi-C Cervical Disc in France in 2004, and in 2013, became the first company to gain U.S. FDA approval for one- and two-level cervical disc replacement. But ZimVie has continued to reach new milestones in cervical disc arthroplasty, including two recent FDA approvals.
In August 2023, the FDA approved a new, smaller profile offering for Mobi-C in the U.S.—a 4.5mm height implant in all of the seven available footprints to address the anatomical needs of the U.S. patient population. The improper sizing of an artificial disc can lead to problems such as prosthesis migration, subsidence, and segmental kyphosis. A 4.5mm height Mobi-C disc allows greater flexibility for surgeons to choose the best fitting artificial disc for patients.
In September 2023, the FDA approved ZimVie’s application for a Mobi-C Cervical Disc Hybrid Investigational Device Exemption (IDE) study, the first such study approved for any medical device manufacturer. This study will follow patients who receive simultaneous cervical disc arthroplasty (CDA) and anterior cervical discectomy and fusion (ACDF) at adjacent levels between C3 and C7. In some cases, the best two-level treatment may be a hybrid construct. This is where the disc replacement and fusion can be completed in one surgery, providing a clinical benefit to patients and surgeons. This study could lead to more patients gaining access to the most appropriate treatment for their anatomy and pathology.