A graveyard full of well-intentioned apps litter tablets and smartphones. The reason: Apps often fail to engage their audience (healthcare professional and patient) at scale and over time. As a result, most apps are used briefly and deleted, or there is poor download and app engagement.
That is the stark reality facing marketing professionals and their promotional agencies looking for innovative ways to deliver high-value information and education to customers about prescription drugs and medical devices. Another stark reality, according to Lisa Drucker, Senior Director, Regulatory Affairs of Celgene: “Mobile apps are probably the most difficult project type to get approved by a promotional review committee.”
That’s why Drucker strongly believes that cross-functional teams must collaborate on a streamlined review process so that mobile apps can be reviewed efficiently for optimal functionality and rapid deployment in the market. “Mobile apps can enhance the safe and effective use of prescription medications by providing disease and safety information and education, along with reminders for appointments, dosing, and refills. In addition, mobile apps can help patients access their prescription medications and devices by providing information on pharmacy home delivery and patient assistance programs.”
To shed greater light on best practices for a streamlined approach to mobile app review, Drucker presented to a diverse audience of stakeholders at the 4th Promotional Review Committee Compliance & Best Practices conference sponsored by ExL Events. As co-presenter, I had the opportunity to construct a roadmap with Drucker for this presentation.
Nailing Down the Mechanics and Mindset for Review Efficiency
Both of us agree that an internal dynamic review system requires efficient/streamlined submission “mechanics” and a proactive, can-do “mindset.” Mechanics and Mindset are equally important to build confidence in delivering compliant promotional apps in the face of well-known challenges. Solutions do exist. See Figure 1.
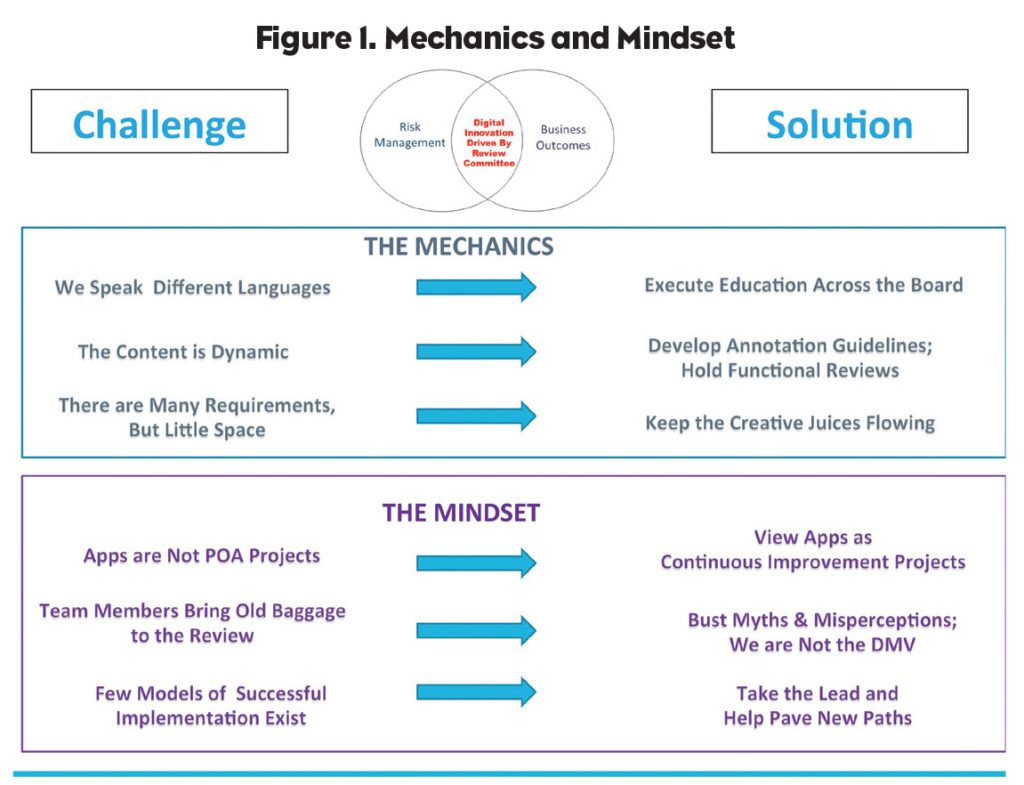 The Mechanics
The Mechanics
Education is vital because we speak different languages
Drucker explains that because mobile app technology is complex and constantly evolving, it’s imperative that the promotional review committee (PRC) understands how the technology works. “Without this basic understanding, fear and uncertainty can result in significant restrictions on the information presented or a failure to think creatively about how regulatory requirements can be satisfied. Equally important: App developers must understand how to navigate the PRC. Concept discussions must be held as early as possible. It’s always helpful for everyone to share the same understanding of key terms.”
Develop annotation guidelines and hold functional reviews to address dynamic content
The importance of a quality submission document to increase reviewer efficiency and save everyone time in the process cannot be underestimated, Drucker emphasizes. This is particularly important given that app content is dynamic and PRC-review processes and FDA submissions are “static” (i.e., PDF screen shots of the app). One best practice, according to Drucker, is to train communications agencies about how the PRC team wants them to annotate the PDF screenshots to describe to reviewers (and FDA, if applicable) how the app functions. This can be accomplished by developing annotation guidelines. For example, if the app contains hyperlinks to a website, the URL that the link directs to should be annotated on the document.
Once the PDF document is approved through the PRC process, it is important to conduct a functional review of the app to confirm that the app performs consistent with the PRC approval. Regulatory reviewers will want to verify prominence of safety information and confirm that the current PI can be accessed through the app.
Keep creative juices flowing to meet regulatory requirements within a small amount of space
All promotional materials must contain a fair balance of risk/benefit, including mobile apps about products where space for risk information communication may be limited. “While it may seem challenging to meet regulatory requirements including those provided by FDA in its digital guidances, or those related to HIPAA regarding the safeguarding of personally identifiable health information, there are proven and creative ways to meet the regulations so everyone needs to keep their minds open,” asserts Drucker.
The Mindset
Following Drucker’s remarks about the mechanics involved in a smoother review process, I followed with best practices on Mindset.
Apps are not plan of action (POA) projects, but rather continuous improvement projects
Unlike web or banner pages, mobile apps are not single point solutions but rather living, breathing transactional service commitments to your customers. Apps are solutions designed to help patients navigate their lives—and their lives aren’t static. As a result, marketing and the PRC must collaborate with the Marketing Department to continually measure, enhance, modify, and seek to improve the HCP or patient app experience.
Increase internal ROI (Don’t come to PRC ready for a fight)
Commercial and Compliance functions (regulatory, legal, compliance) must work together collaboratively as partners to achieve market success. Creating a path forward for alignment drives the top and bottom line, leading to greater market competitiveness and reduced risk, while serving patients, prescribers, and public health.
When promotional review teams partner with marketing professionals early in the development of digital tools, a company greatly enhances its internal ROI. Internal ROI reflects the efficient use of time and money spent internally preparing a digital tactic for use externally. (See article on Internal ROI, PM360, September 2015: www.pm360online.com/increase-internal-roi-for-digital-tactics-2.)
Lead the way and help drive the business
Promotional review team members can demonstrate their value to the business by proactively researching mobile app systems and best practices that address existing compliance requirements, and perceived compliance barriers. These include: 1) documentation formats that facilitate brand-specific balance and disclosure and simplified 2253 OPDP submissions; 2) HIPAA security protections for patient data; 3) capability for live app edits without App Store delays; and, 4) provisions for live app promotional review at project start, to better assess actual app functionality.
It’s possible to “pre-test” an app for compliance before it’s been constructed in three ways:
- Comparing desired app key functionalities to existing apps that can be reviewed live in PRC meetings.
- Actual live pre-test of desired app functionalities using custom solutions from digital agencies.
- Actual live pre-test of actual apps in a secure testing environment such as with the Mobile Health Library (MHL), the only HIPAA compliant multi-brand app platform supporting HCPs and patients.
Ultimately, reviewers have many avenues to demonstrate their value to commercial colleagues to manage risk and drive business outcomes.
Disclaimer: The views and opinions expressed in this article are those of the presenter and may not reflect the policy or position of Celgene Corporation.



