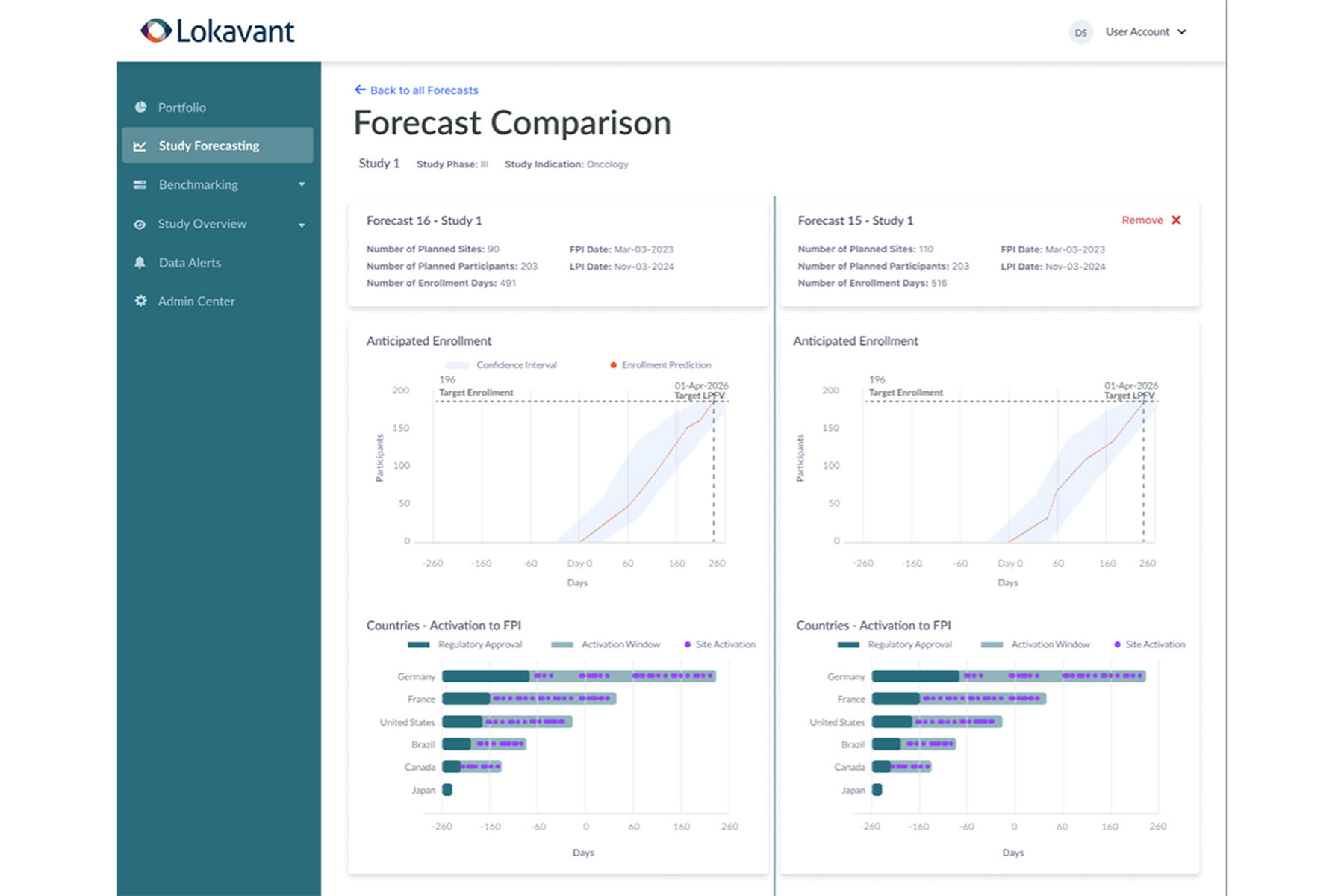
Lokavant’s Study Planning Solution
Identifying the Right Conditions for Clinical Trials
To determine clinical trial feasibility, sponsors typically look at sites’ historical enrollment performance, resources, and experience. However, sponsors and contract research organizations (CROs) have struggled to accurately determine site success. As a result, 70% of clinical trials experience study start-up delays, 80% don’t complete the enrollment phase on time, and 45% miss their projected end dates.
In August 2023, Lokavant, the clinical trial intelligence platform company, launched its AI-powered Study Planning Solution—driven by proprietary historical trial data from more than 2,000 studies involving more than 14,000 investigators, 12,000 healthcare institutions, and real-world data (RWD) sources. The new solution empowers clinical trial sponsors and CROs to accurately identify the right number, location, and mix of sites for key performance and diversity milestones, as well as other feasibility goals for optimum study performance.
For example, Lokavant gathered five years of data on chronic obstructive pulmonary disease (COPD) to accurately identify the most efficient and inefficient trial sites for future respiratory trials. Analysis revealed that sponsors and CROs could reduce the number of sites needed for a study by 18% to 36% and still hit patient enrollment targets. Given the average cost to activate sites regardless of patients accrued is $50,000 and the per-site cost of site monitoring—which accounts for about 14% of total clinical trial expenditures—optimizing site selection leads to significant cost and time savings.
The Study Planning Solution will be generally available in Q4 2023 as a standalone product or add-on module to Lokavant’s flagship Clinical Trial Intelligence Platform.