Understanding the “why” before the “how” as Med Affairs charts the course for pre- and post-launch strategies.
In the rapidly evolving landscape of healthcare, where breakthrough therapies and treatments emerge at an unprecedented pace, conventional boundaries are rapidly shifting.
Medical Affairs is at the forefront of this paradigm shift. With a focus on patient-centricity, data-driven insights, and collaborative partnerships across the healthcare ecosystem, it’s redefining the playbook for market shaping and building advocacy for a product pre- and post-launch. The role of Medical Affairs transcends mere facilitation.
It covers the product lifecycle in its entirety by delivering innovative approaches to anticipate and address the needs of patients, healthcare providers, and payers alike.
Making the Case for Early Medical Affairs Involvement
Early involvement of Medical Affairs in the product lifecycle is not just beneficial—it is mission critical. In the swiftly changing realm of advanced therapies like biosimilars, rare disease molecules, and cell and gene therapies, a strong scientific interpretation
and orientation is essential. Teams are stepping up to this challenge, actively shaping the trajectory of new interventions right from their inception.
Gone are the days when Medical Affairs merely acted as a liaison between pharmaceutical companies and healthcare professionals (HCPs). Today, teams are indispensable strategic partners, deeply involved in crafting the very foundation of a product’s journey. From defining the target product profile to shaping clinical endpoints and devising asset strategies to crafting scientific narratives, the value of Medical Affairs permeates every aspect of product development.
The function is increasingly adopting a collaborative mindset, forging partnerships with academia, advocacy groups, and healthcare organizations to co-create solutions that address unmet medical needs. By engaging with stakeholders early in the development process, professionals can ensure the voice of the patient is central to decision-making, resulting in more meaningful and impactful interventions.
Driving an Integrated Evidence Strategy
Evidence-based decision-making and differentiation are of paramount importance for HCPs who are flooded with therapy options or are circumspect to adopt a new therapy. Similarly, payers face a dilemma as they frequently hesitate to embrace emerging treatments because of the lack of consistent, longstanding, and customized evidence.
The imperative for a robust evidence generation and dissemination strategy tailored to the diverse needs of stakeholders looms large. With a nuanced understanding of the field, Medical Affairs teams emerge as the natural leaders in spearheading evidence gap identification, stakeholder assessment, and evidence generation endeavors, right from inception to post-launch phases. Post-launch, the role of Medical Affairs groups continues to evolve, focusing on driving patient outcomes and optimizing
the use of therapies in real-world settings. Through initiatives such as post-marketing surveillance, pharmacovigilance, and medical education programs, Medical Affairs teams are committed to ongoing monitoring and support to ensure the safe and effective use of medications.
Building a Medicalized Engagement Strategy
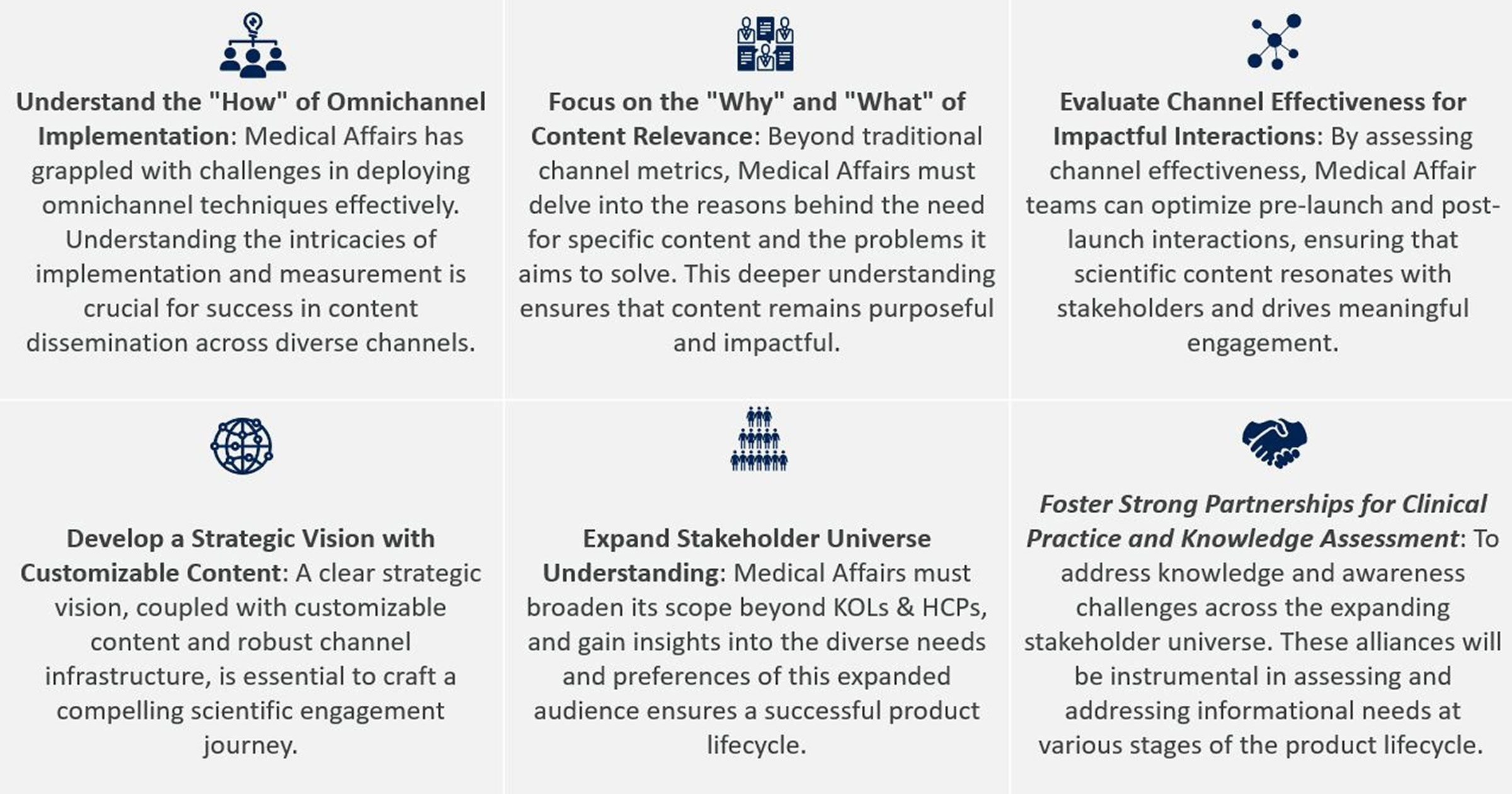
Through the course of the product lifecycle, effective content development and dissemination is an important goal, and an omnichannel approach is the way forward. A clear understanding of the steps highlighted below will help the development of strong scientific partnership-based engagements with stakeholders.
The journey of a product’s lifecycle is complex, from inception to post-launch, making the creation and distribution of compelling content even more vital. To navigate this effectively, Medical Affairs leaders must understand these six essential steps to pave the way for strong scientific partnership-based engagements with stakeholders across the ecosystem.
Clinical-Medical-Commercial Collaboration
Within the intricate web that is the healthcare ecosystem, stakeholders perceive pharmaceutical companies as singular entities, irrespective of internal divisions between Medical Affairs, clinical and commercial teams. Presenting a united front necessitates seamless collaboration and alignment across all divisions of the organization.
Medical Affairs assumes a pivotal role in orchestrating cohesive stakeholder engagement that spans the entire product lifecycle. By actively partnering with clinical and commercial teams, it can enable partnerships between all functions that transcend a traditional siloed approach. This will require the organization to establish appropriate capabilities, systems, and processes, enhancing transparency and visibility of goals, objectives, and tactics within each function. Ultimately, the aim is to foster a unified brand, characterized by ‘One Goal, One Voice, One Message’ for the pharmaceutical company.
Harnessing the Power of Medical Insights
Medical insights are a goldmine of invaluable information, offering first- hand knowledge of market dynamics and stakeholder needs throughout the product lifecycle. They serve
as the guiding light for designing and refining content, engagement strategies, and pre- and post-launch initiatives in real-time. Drawing from sources such as Medical Information, Medical Education, Publications, and Field Medical teams, these insights offer a panoramic view of trends, burning questions, hot topics, and complex queries.
The transformative potential of medical insights extends beyond Medical Affairs, influencing clinical, commercial, and organizational strategies. By harnessing this wealth of information, companies gain a competitive edge, adapting swiftly to evolving market demands and stakeholder expectations.
At the heart of this strategic transformation lies a comprehensive Insights Management Framework. Supported by the right systems and training modules, this framework empowers Medical Affairs to provide strategic readiness, positioning organizations for success in an ever- changing healthcare landscape.
Decrypting the Patient Journey from a Medical Lens
Understanding and addressing patient needs is an area where Medical Affairs will play a more active role in the future. Areas including trial enrollment or Managed Access Programs, coordination with Patient Advocacy Groups to imbibe patient input into treatment development, product access post-launch, and RWE support for added indications will become the mainstay for Medical Affairs as we move ahead. It is set to play a more proactive role in deciphering the intricate pathways of the patient journey, leveraging its unique blend of expertise in communication, disease understanding, and evidence-based medicine.
Data as the Accelerator for Medical Affairs’ Role Evolution
At the heart of the transformation of Medical Affairs is the power of data. From the initial stages of drug development to post-launch activities, the use of data, analytics, and insights will redefine how Medical Affairs demonstrates its impact. Greater reliance on data to enhance stakeholder engagement efficacy will drive the need for pharmaceutical organizations to propel data-driven techniques across Medical Affairs functions such as Medical Communications, Medical Information, Medical Education, etc.
An honest understanding of Medical Affairs situations and needs will be essential to ensure data-driven decision-making. Key strategies such as segmentation techniques, omnichannel approaches, and the implementation of insights tagging and analysis systems are essential components in this journey.
Moreover, the establishment of standardized data protocols and integration initiatives is imperative to ensure seamless adoption and utilization of Medical Affairs data across the organization. As the advent of Artificial Intelligence (AI) continues to permeate the healthcare sector, its role within Medical Affairs becomes increasingly significant. AI presents unparalleled opportunities to analyze vast amounts of structured and unstructured data rapidly, unlocking invaluable insights to drive strategic decision-making.
From the initial stages of drug development to post-launch activities, the use of data, analytics, and insights will redefine how Medical Affairs demonstrates its impact.
The Time Is Now to Embrace Global Medical Affairs Across Commercialization
Now is the time for global teams to embrace the role of Medical Affairs teams in the commercialization process.
Medical Affairs leaders must delineate a robust lifecycle strategy, underpinned by comprehensive support in people, processes, and technology. Most importantly, this global function needs to be integrated as “one team” with “one goal” for the success of the product and access for the patient.
Central to this transformation is the establishment of a tailored KPI framework, equipped with metrics
that capture the multifaceted impact of Medical Affairs across the product lifecycle. Metrics must be intuitive yet insightful, enabling stakeholders to appreciate the value of sustained Medical Affairs involvement.
As we embark on the journey towards personalized medicine and personalized customer needs, the role of Medical Affairs in empowering the healthcare ecosystem to make informed decisions cannot be overstated. Through its commitment to therapeutic significance, scientific advocacy, and sustained customer engagements, Medical Affairs stands as a beacon of excellence in guiding the journey for patients where they receive the care they deserve.