Patient Pages: First Contraceptive App Under Fire from Users
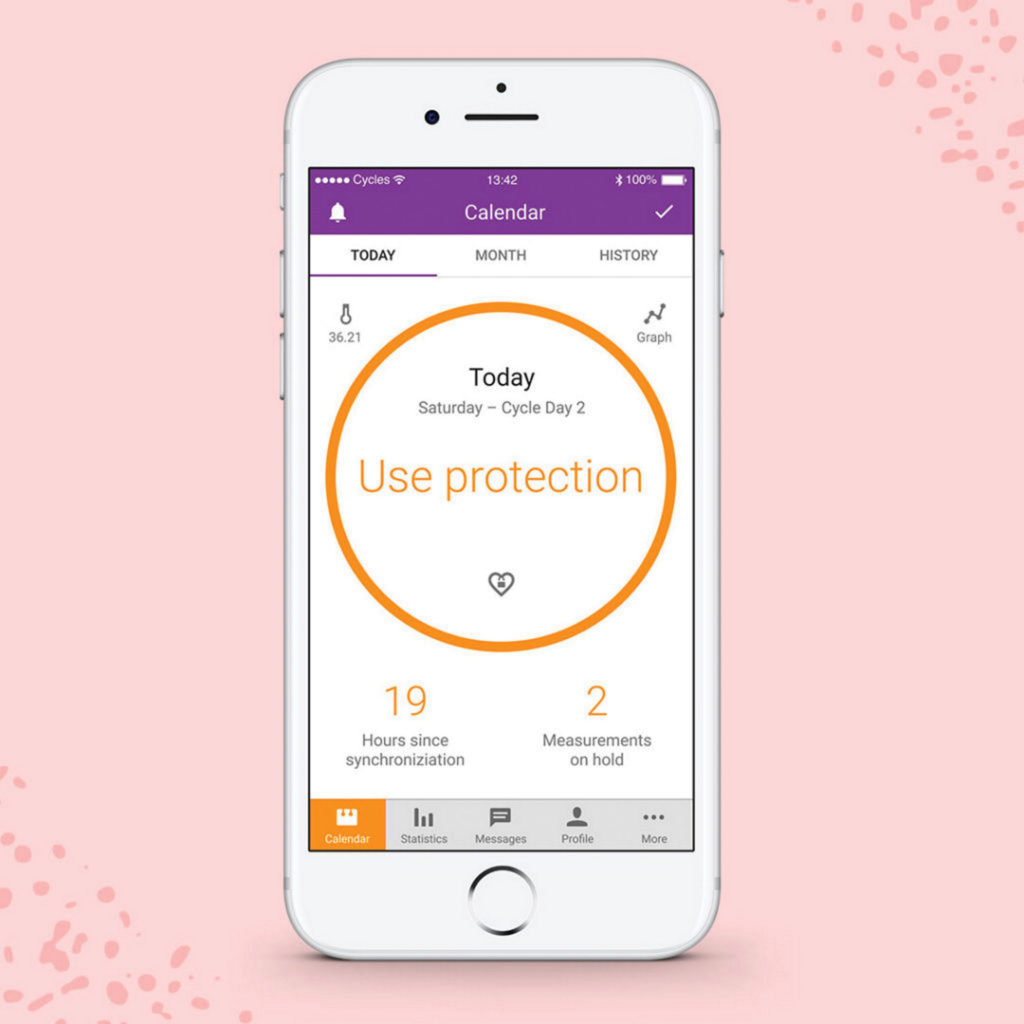
Natural Cycles app, designed to track fertility and help women prevent pregnancy, is the first app to be approved for marketing as a contraceptive by the FDA. The effectiveness of the app as a contraceptive is receiving heavy backlash, especially from women who say they trusted the marketing and ended up pregnant.
While Natural Cycles claims that its effectiveness has been proven to be 93% for typical use and 99% for perfect use—in three published studies—critics say that fertility awareness studies are mediocre at best and are suspicious as to why the CDC marks it as the most effective contraceptive option.
Some believe it is a contraceptive tactic used by fertility awareness centers to promote abstinence from sex when fertile—rather than more contraception methods. Major concerns were brought up earlier this year when Södersjukhuset, one of the largest hospitals in Stockholm, reported that at least 37 women sought abortions after using Natural Cycles. A spokesperson for Natural Cycles confirmed the report, adding that, “Unwanted pregnancies are an unfortunate risk with any type of contraception.”
Natural Cycles caters to the one in five women who would prefer to use this method over hormonal medications, which requires users to take their temperature with a basal body thermometer each morning and combine it with data about their menstrual cycle dates to predict ovulation cycles. The app glows red if there is a risk of pregnancy and turns green if it is safe to have sex.
Trend Setting: CVS Rides the Telehealth Wave

MinuteClinic, the CVS retail medical clinic, is entering the virtual health game by launching MinuteClinic Video Visits through the CVS pharmacy smartphone app. Patients with minor illnesses and injuries, including minor wounds, skin conditions, or colds, can virtually see a healthcare provider on their phone, 24 hours a day.
CVS finds that 95% of their patients are happy with the convenience of their telehealth services as well as the quality of care they received. Troyen A. Brennan, MD, Executive Vice President and Chief Medical Officer of CVS Health, said in a statement, “At CVS Health, we’re committed to delivering high-quality care when and where our patients need it—and at prices they can afford. Through this new telehealth offering, patients now have an additional option for seeking care that is even more convenient for them.”
Using Teladoc’s digital platforms, CVS patients 2-years-old and up can enter a video consult with a MinuteClinic physician based on a health questionnaire that matches them to one of the board-certified healthcare providers licensed in their state. A MinuteClinic Video Visit costs $59, and insurance coverage is expected to be added in the coming months.
Discoveries/Innovations: CellMax Set to Revolutionize Cancer Detection

CellMax is changing how we detect colorectal and prostate cancer with a biometric platform that detects pre-cancer and earliest stage cancer with 84% to 88% accuracy. CMx detects circulating tumor cells (CTCs) in a simple, globally affordable blood test, which has been awarded six U.S. patents. “In the past, finding CTCs was not possible in pre-cancer and early stage cancer, as the cells numbered too few to accurately identify in the bloodstream,” said Shai Friedland, MD, Chief of Gastroenterology & Hepatology, Stanford University School of Medicine, in a statement. “The CellMax CMx platform’s ability to achieve high sensitivity for pre-cancerous colorectal lesions, while remaining cost effective and convenient, is notable. The CMx platform positions CellMax Life’s CTC test to potentially become a standard option for the 100 million Americans over the age of 45 who are eligible for colorectal cancer screening.” Traditional methods used for early detection often missed CTCs and left them unnoticed until advanced stages are upon the patient.
Brand Beat: Migraine Sufferers Unaware of New Drug
Despite the significant step toward making debilitating migraines manageable with FDA approval of calcitonin gene-related peptide (CGRP) therapies, two-thirds of migraine sufferers remain unaware of this new and effective treatment. According to a “Migraine in America 2018” survey, only a third of people with the condition (roughly 37 million) are even aware of CGRPs—the first medication developed specifically for the preventive treatment of migraine—and of that third, nearly half still had questions and concerns about the treatment.
CGRP is a molecule that is synthesized in neurons, the nerve cells in the brain and spinal cord, and functions as a vasodilator. While 79% of aware sufferers are “very likely” to use CGRPs, they are woefully uninformed about affordability and cost, insurance coverage, side effects, how they work, when they will be available, their effectiveness, and how they will interact with current treatments.
Tim Armand, Health Union President and Co-founder, said in a statement that the results of the survey are consistent with what he’s witnessed on Migraine.com. “People are very excited about what these treatments could potentially mean for their condition management, but—plain and simple—they have a lot of questions and concerns.” This lack of awareness may be coming from the provider’s own lack of information. Nineteen percent of respondents heard of CGRPs from their doctors, while more than half learned of them from internet sources such as Migraine.com.
TeleMed Text: Breakthrough Bilingual App for U.S. Providers and Patients
Health-tech startup Hoy Health has met the needs of English- and Spanish-speaking patients who seek accessible healthcare in their own language. The newly launched HoyDOC smartphone app is a telemedicine app that connects Spanish- and English-speaking patients with appropriate providers. Particularly useful for the underserved with limited access to healthcare, the app helps to solve issues with choosing the time and location of a doctor’s appointment, which comprises 80% of the reasons this population does not always receive the medical attention it needs.
“The HoyDOC mobile app gets us one step closer to providing a complete primary health bilingual ecosystem and one step closer to fulfilling our mission to provide accessible and affordable healthcare to everyone, everywhere,” said Mario Anglada, Chief Executive Officer, Hoy Health LLC, in a statement. So far, the HoyDOC mobile app allows its users to consult with a provider from wherever they may be and store their medical records in a HIPAA- and HITECH-compliant platform.
FDA Update
Drug Approvals
Alnylam Pharmaceuticals received FDA approval for Onpattro, a first-ever treatment for patients with polyneuropathy caused by hereditary transthyretin-mediated amyloidosis (hATTR), a rare and often fatal genetic disease characterized by buildup of abnormal amyloid protein in peripheral nerves, the heart, and other organs. Onpattro is the first-in-class of drugs called small interfering ribonucleic acid (siRNA) treatment. The medication is designed to target the causes of the disease, inhibiting or reversing the genetic drivers in RNA, allowing for a potential reversal of the condition.
Amicus Therapeutics’ Galafold was approved as the first oral treatment for adults with Fabry disease. Galafold increases the activity of a deficient enzyme in the patient’s body that leads to buildup of certain fat in blood vessels, the kidneys, the heart, the nerves, and other organs. The rare genetic disorder causes progressive kidney disease, cardiac hypertrophy, arrhythmias, stroke, and early death.
Teva received FDA approval for the first generic competitor of Mylan’s epinephrine injecting device, EpiPen. The unnamed and yet unpriced generic rival comes a year after Mylan hiked prices from $50 to $600 while holding a tight patent on the combination epinephrine injection pen device, which costs a dollar to manufacture. After suing Teva in 2009 for patent infringement, it is the only company to finally manage to make an approved device that can be used interchangeably with Mylan’s EpiPen.
Breakthrough Therapy
Roche and Novartis received a breakthrough therapy designation for Xolair to prevent severe allergic reactions in people with food allergies after exposure to one or more foods. The designation was given based on seven clinical studies reviews of the drug’s safety and efficacy. Roche is also collaborating with the NIH and the Consortium of Food Allergy Research to begin a pivotal study of Xolair for treatment of multiple food allergies.
Medical Devices
The first patient-controlled, reversible, long-lasting birth control device has been approved by the FDA. TherapeuticsMD developed ANNOVERA as a vaginal contraceptive system that can be inserted by the user and removed for seven days each month without replacement for a year. The small, flexible ring prevents ovulation as the woman inserts and removes the device for four week cycles at her own discretion.