Patient Pages: Conveying Truth About Vaccines

New York City declared a health emergency and ordered mandatory measles vaccinations this past month after two children without the vaccination developed the disease and caused the largest outbreak of the disease, previously declared eliminated, in years. This, and more than 460 cases occurring across the U.S., are occurring amid a brutal debate about the safety of vaccines, seemingly spurred by misinformation spread on influencing sites such as Facebook, Amazon, YouTube, and others.
Doctors and other health-aware individuals and scientists are becoming increasingly frustrated with the inability to communicate the truth about vaccines due to the hostility surrounding the subject. Board member of the American Academy of Family Physicians, Dr. Leonard Reeves, explains that while conversations with trustworthy providers are vital in such decision making, “when we’re spending more than half of the visit discussing the most evidence-based thing that we do, that’s a problem.”
Verywell interviewed 1,000 patients to better understand the vaccine conversation and design Healthy Conversations, a new approach to helping people best articulate when broaching sensitive health issues. The tool simulates a discussion (similar to a text message) you might have about a tough topic. Specific personas are identified, and then common questions, answers, and concerns related to the topic are addressed with conversational sensitivity and medical accuracy. The goal is to prepare a script and educate people, even HCPs, on how to have more fluid and productive conversations for better overall outcome.
Trend Setting: Amazon’s Alexa is Now HIPAA Compliant

This month, Amazon began inviting certain developers to create HIPAA-compliant “skills” for their Alexa smart speakers, which are programs that allow users to ask Alexa personal medical questions. The challenge to pharma marketers becomes making connected skills useful to their work, as well as to the patient. This means it won’t be good enough to design skills that allow Alexa to track data—developers will need to find ways to send this data to HCPs, pharmacies, and other useful resources.
Anne Weiler, CEO of Wellpepper, whose Sugarpod voice application won the Merck-sponsored Alexa Diabetes Challenge in 2017, says that pharma could also team up with partners on the platform. “Patients are looking for something comprehensive whereas pharma can really only talk about that one piece of it that surrounds the drug,” she said in a statement, “so plugging into other skills is probably going to be one of the more interesting uses for them.”
Amazon has already launched several medical skills. One such skill, from Cigna developers, allows eligible employees to manage their health improvement goals and earn wellness incentives. Another comes from Livongo, a diabetes tech developer, that lets members ask Alexa for their last blood sugar reading. Express Scripts’ skill is one that currently offers updates on prescription delivery or allows for appointment making.
TeleMed Texts: Facebook Bot Tackles Painful Sex
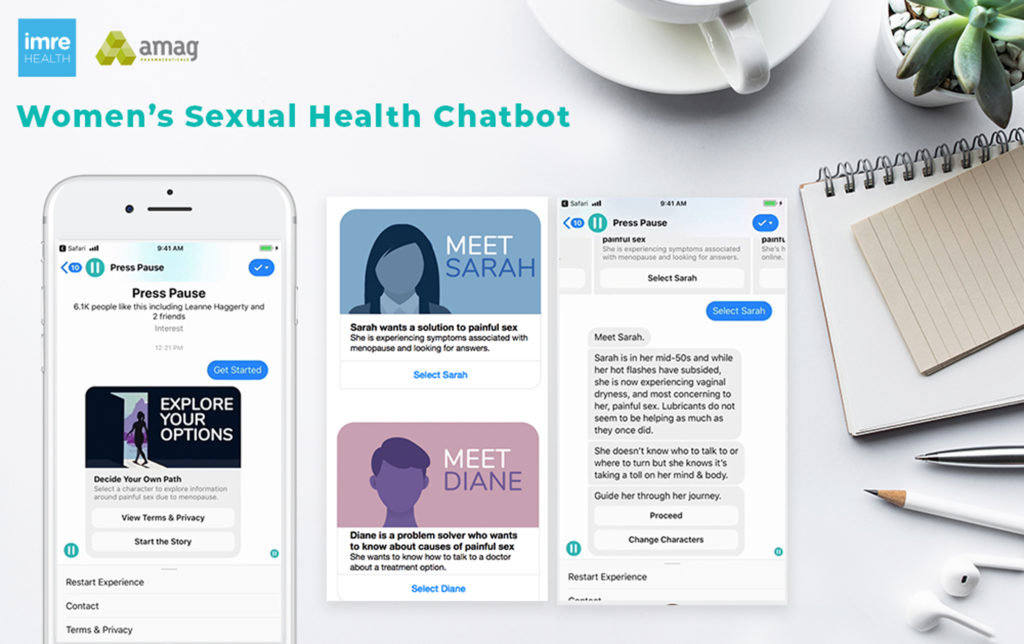
AMAG Pharmaceuticals and imre Health teamed up to create a chatbot, living in the Facebook messenger app, to help women have the awkward conversation about an issue that needs to be discussed: Painful sex. Pain during sex, a condition called dyspareunia, affects up to 50% of menopausal women, but few want to talk to their doctors about it, fearing they won’t be taken seriously.
imre, the empathetic healthcare marketing agency, developed a chatbot to give women an anonymous, but interactive place to learn about the condition. AMAG, needing a way to further awareness not only of the legitimacy of the condition, but also of their novel solution, Intrarosa, a prescription vaginal insert for use in treating painful intercourse caused by changes during menopause, provides the bot’s content knowledge.
The novel device aims to break through the stigma surrounding dyspareunia and combat misinformation; imre found that women who bring this problem up to doctors are often given mistreatment or no treatment at all. Some patients have even been told to take a bath or drink some wine to help loosen them up—certainly not a solution to a real medical problem. Beyond offering anonymity, a Facebook-based app works well in this arena because menopause is a growing topic online. Nearly 53,000 posts are made each month. Seven out of 100 menopause posts are about VVA (dyspareunia is a form of VVA) and four out of 100 menopause posts are about painful sex. This shows that women of this demographic are turning to social media for answers when they could not be found in the traditional doctor’s office setting. imre is now filling a market need for these patients, giving them an opportunity to improve their health and quality of life.
Discoveries/Innovations: CRISPR Tech May Be Able To Combat Alzheimer’s
We’ve known that our immune system destroys damaged neurons and protein plaques in our brains and that all important microglia clean up their debris via phagocytosis. As we age, microglia’s effectiveness naturally diminishes and we can accumulate toxic proteins like beta amyloid, a hallmark of Alzheimer’s disease. Researchers from Stanford University have now drawn a link between this metabolic build up and cognitive decay, while also revealing a promising process for reversing the aging of microglia.
Using CRISPR-Cas9 and RNA sequencing, the Stanford scientists identified CD22—a B-cell receptor that regulates immune responses and is a negative controller of microglial phagocytosis. CD22, out of the 3,000 gene-encoding proteins that they judged could be targeted by drugs or that had already been the focus of drug development for Alzheimer’s and related cognitive degeneration, is the only one that regulates microglia’s phagocytosis and whose activity in microglia substantially increased with advancing age.
In studies, suppressing CD22 appears to improve cognitive function in aged mice.
DC Dispatch: Sugar Labeling Could Reduce Healthcare Cost
The FDA has set a mandatory sugar labeling policy for packaged food and beverages that experts believe will have significant financial benefits for the U.S. healthcare system, along with health benefits for the overall society. The policy, which will take effect in 2020, was analyzed by researchers at the Friedman School of Nutrition Science and Policy at Tufts University and the University of Liverpool to produce the first estimate of the potential health and economic impacts of the new label.
The study estimates that the additional sugar label could prevent or postpone nearly one million cases of cardiometabolic disease, including heart disease, stroke, and type 2 diabetes, over a 20-year period. After considering the possible industry reformulations to reduce added sugar content in packaged foods and beverages, the label could prevent or postpone nearly three million cases of cardiovascular disease and diabetes over the same time period.
Specifically, the sugar label could prevent or postpone 354,400 cases of cardiovascular disease and 599,300 cases of diabetes, which would save $31 billion in net healthcare costs and $61.9 billion in societal costs. Policy costs were estimated to be $1.7 billion. After considering industry reformations, this number rises to $57.6 billion in net healthcare costs and $113.2 billion in societal costs. Policy costs, including industry reformulation costs, were estimated to be $4.3 billion.
“The added sugar label is an important policy step toward reducing consumption of foods and beverages with high added sugar contents, improving health, and lowering healthcare spending,” Renata Micha, RD, PhD, the study’s co-senior and corresponding author, said in a statement. “These findings have important implications for individuals, policy makers and the food industry alike.” Labeling the grams and percent of daily value of added sugar content was among changes the FDA implemented. The goal is to help consumers limit calories from added sugar in accordance with the 2015-2020 Dietary Guidelines for Americans.
FDA Update
Drug Approvals
The FDA grants accelerated approval to Janssen’s Balversa, a treatment for adult patients with locally advanced or metastatic bladder cancer that has a type of susceptible genetic alteration known as FGFR3 or FGFR2, and that has progressed during or following prior platinum-containing chemotherapy.
EMD Serono receives approval for Mavenclad, oral tablets that treat relapsing forms of multiple sclerosis (MS) in adults, especially a relapsing-remitting disease.
Amgen’s Evenity, is a new treatment specifically indicated for osteoporosis in postmenopausal women at high risk of bone fractures. The injectable therapy is administered twice by a physician, one immediately following the other. Evenity is a monoclonal antibody that blocks the effects of the protein, sclerostin. It works mainly by increasing new bone formation, but this effect wanes after 12 doses. The new drug may increase the risk of heart attack, stroke, and cardiovascular death, so it’s important to eliminate those who have had a heart attack or stroke within the previous year.
AbbVie’s Skyrizi, an interleukin-23 (IL-23) inhibitor, was approved for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. It is the first IL-23 drug to hit the market since Johnson & Johnson’s Tremfya.
Med Device Approvals
The FDA approved marketing of Channel Medsystems’ Cerene Cryotherapy Device, an endometrial ablation device that uses a freezing agent to destroy the lining of the uterus (or endometrium) to reduce heavy menstrual bleeding. The probe releases a freezing agent to reduce bleeding in pre-menopausal women with benign, heavy menstruation who do not want to become pregnant in the future.







