Med Device: Aortic Valve System Shows Efficacy and Durability

A recent study involving Medtronic’s CoreValve system yielded promising results in patients with severe aortic stenosis (a heart valve disease in which the aortic valve doesn’t open properly). CoreValve, which was FDA approved in January 2014 for patients considered to have an extreme risk for open heart surgery, is a device implanted in the aortic valve that is designed to self-expand to prevent unwanted leakage and optimize blood flow.
According to the study, after patients were treated with the CoreValve system, significant improvements in quality of life were seen at one year through four years, with 74% of patients improving after attending at least one New York Heart Association class. The study also showed long-term durability and excellent clinical performance with no structural valve dysfunction at four years. The CoreValve CE Pivotal Study was the first rigorous evaluation of the system and one of the first studies to report data for transcatheter valve durability.
DC Dispatch: Mental Health Services Receive Huge Investment
After decades of budget cuts, mental health services are finally receiving the financial support they deserve. With the recent passage of legislation that approved $900 million in funding for services covered under the Excellence in Mental Health Act championed by Senators Debbie Stabenow (D-MI) and Roy Blunt (R-MO) along with Representatives Doris Matsui (D-CA) and Leonard Lance (R-NJ), approximately 240,000 people will be able to receive behavioral health services.
As part of the Medicare SGR Repeal bill, the legislation establishes a two-year demonstration program in eight states that will offer mental health and substance abuse treatment services. Also, troops serving in Iraq and Afghanistan can now access treatment for service-connected mental disorders.
Therapeutic Talk: Phase III Studies Show Reduction in LDL Cholesterol
More good news in the field of cardiovascular medicine and for biopharmaceutical manufacturer Amgen. Two crucial Phase III studies (GAUSS-2 and LAPLACE-2) conducted on the company’s novel investigational cholesterol-lowering medication, evolocumab (AMG 145), showed significant reduction in low density lipoprotein (LDL), between 37-39%, compared to another medication, ezetimibe, in patients with high cholesterol who can’t tolerate statin drugs, and between 55-76% compared to a placebo when taken together with statins.
Unlike statins, which inhibit the HMG-CoA reductase enzyme, both evolocumab and ezetimibe are selective cholesterol-absorption inhibitors. Evolocumab inhibits protein convertase subtilisin, which reduces the liver’s ability to remove LDL from the blood. In both studies, adverse events were less prevalent (5% and 1.5% in the combined groups). The most common adverse effects were headache, muscle spasms and pain in the extremities.
Trend Setting: Hispanics Have Limited Understanding of “Obamacare”
A Spanish language online survey was conducted by Hispanic health company and website, HolaDoctor, to determine the Hispanic community’s opinion of the Affordable Care Act (ACA) and their plans to purchase insurance. The term “Obamacare” was used in the survey as it is most well known within the Hispanic community.
The survey revealed that 62% of respondents were uninsuranced, and 57% of them had intentions to purchase a plan via “Obamacare.” Of those without insurance, 77% said they would purchase a plan via the marketplace. Nearly half said that they would not purchase a plan due to low incomes.
According to Dirk Schroeder, Executive VP and Chief Medical Officer of HolaDoctor, the data shows that Hispanics are open to “Obamacare,” but still need more information to understand the law and enroll in exchanges.
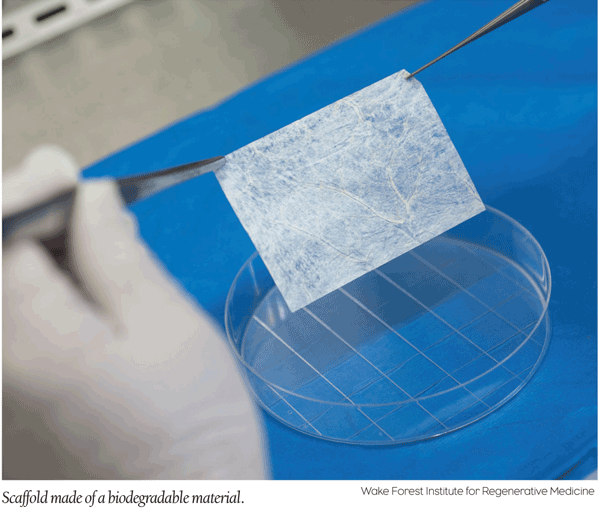
Discoveries/Innovations: Scientists “Grow” Cartilage and Reproductive Organs
Tissue engineering, typically used to repair burned skin or torn muscles, took a giant leap forward recently when scientists from the U.S., Mexico and Switzerland successfully grew and implanted both nasal cartilage and reproductive organs in patients, according to two studies published in The Lancet—with virtually no complications, even years later.
One of the studies focused on four teenagers born with Mayer-Rokitansky-Küster-Hauser syndrome, a condition that results in a missing or deformed uterus, an absent or ill-formed vagina or another dysfunction, who received new reproductive organs. In the second study, five elderly patients who lost large areas of nasal tissue to cancer surgery received engineered cartilage implants.
While tissue engineering is not new, creating larger, complex organs is. To create the reproductive organs, scientists first built a 3-D scaffold (made of a material that eventually dissolves in the body) sized to the dimensions of the patients’ missing or damaged organs as a framework. On that, they placed swatches of patients’ muscle and tissue and “seed” cells that spread across the surface of the scaffolds and are grown for a few weeks in the lab. After an implant, these organs actually grow with the body. Engineered cartilage is created in nearly the same way—and the second study reports that the nasal structure, function and aesthetics of the five patients remained intact.
Studies involving larger groups of patients will need to be done to confirm these remarkable results, but scientists hope that a much broader group of patients will benefit from these findings in the future.
FDA Update
New Medical Device EAP Proposed
The FDA recently proposed a new program to provide earlier access to high-risk medical devices that are intended to treat or diagnose patients with serious conditions whose medical needs are unmet by current technology. The proposed Expedited Access Premarket Approval Application for Unmet Medical Needs for Life Threatening or Irreversibly Debilitating Diseases or Conditions (“Expedited Access PMA” or “EAP”) program allows earlier and more interactive engagement with FDA staff. While other existing device programs have focused on reducing the time for the premarket review, EAP also seeks to reduce the time associated with product development.
New Approvals
Swedish Orphan Biovitrum AB (Sobi) and partner Biogen Idec received FDA approval for Alprolix (Coagulation Factor IX (Recombinant), Fc fusion protein). The medication is indicated for the control of bleeding episodes, perioperative management and routine prophylaxis in adults and children with haemophilia B. Abbot was granted FDA approval for its Supera Stent, indicated for the treatment of Peripheral Artery Disease (PAD). Bristol-Myers Squibb’s diabetes drug Farxiga (dapagliflozin) also received approval as an oral treatment for type 2 diabetes mellitus.
Warning Letters
Having failed to initiate corrective actions at its Cork, Ireland, manufacturing site, the FDA issued a warning letter to GlaxoSmithKline that threatened to ban materials produced at the plant, which operates under the company name SmithKline Beecham. According to the Dublin-based news website, RTÉ, the plant produces the active ingredients for the antidepressant Paxil and was previously found to have contamination issues. Further, the FDA accuses the drug manufacturer of continuing to distribute its product even after it learned that the product could contain contaminants.