Therapeutic Talk: Eradicating Sickle Cell Disease
 Novartis and the Bill and Melinda Gates Foundation are teaming up to create a sickle cell disease therapy that is an in vivo gene therapy. Sickle cell disease is caused by a gene mutation that largely affects African people. While therapies, including gene therapies, for sickle cell disease are in development, they are all ex vivo, meaning cells are extracted from the patient, treated, and reinjected. Ex vivo processes require manufacturing facilities and hospital infrastructure that many patients in Africa cannot access.
Novartis and the Bill and Melinda Gates Foundation are teaming up to create a sickle cell disease therapy that is an in vivo gene therapy. Sickle cell disease is caused by a gene mutation that largely affects African people. While therapies, including gene therapies, for sickle cell disease are in development, they are all ex vivo, meaning cells are extracted from the patient, treated, and reinjected. Ex vivo processes require manufacturing facilities and hospital infrastructure that many patients in Africa cannot access.
Along with supporting the development phase, the Gates Foundation will help fund global access to any treatment outcomes of the partnership. “Gene therapies might help end the threat of diseases like sickle cell, but only if we can make them far more affordable and practical for low-resource settings,” said Trevor Mundel, PhD, President of Global Health at the Gates Foundation, in a statement. “What’s exciting about this project is that it brings ambitious science to bear on that challenge. It’s about treating the needs of people in lower-income countries as a driver of scientific and medical progress, not an afterthought.”
Novartis aims to make the quick and convenient in vivo therapy a one-dose treatment that can be administered to patients who cannot travel to treatment facilities often or at all. Once the medicine shows promise, the company will prioritize issues of access and distribution in low- and middle-income countries.
Doctor Docs: Doctor Visit Frequency Continues to Fluctuate
 Fewer people were visiting their doctors when the pandemic first hit the U.S. last year, with visits to ambulatory care providers declining by nearly 60% last April, according to a report by Phreesia and Harvard University. But by October 2020, despite HCPs continuing to offer telemedicine appointments, Phreesia reported office visit volumes returning to baseline with a decline in telehealth visits.
Fewer people were visiting their doctors when the pandemic first hit the U.S. last year, with visits to ambulatory care providers declining by nearly 60% last April, according to a report by Phreesia and Harvard University. But by October 2020, despite HCPs continuing to offer telemedicine appointments, Phreesia reported office visit volumes returning to baseline with a decline in telehealth visits.
More challenges arose during the holiday season when COVID cases were on the rise across the U.S. HCPs had to maintain safe standards for seeing patients that might be exposed to or diagnosed with the virus. While most offices were able to maintain their weekly number of offices visits, the number of pediatric doctor visits dropped drastically. The dip in pediatric doctor appointments was especially surprising in the winter, when there is normally a sharp increase. Pulmonology, physical medicine, and cardiology patients also started skipping more visits at the end of 2020, while behavioral health saw a spike in telehealth appointments made.
Patient Pages: What Does Pharma’s Reputation Boost Mean?
The pharmaceutical industry has had a drastic turnaround in reputation due to the COVID-19 pandemic. Last year, only about a third of the U.S. population viewed the industry positively while polls from February 2021 report 62% of the U.S. population rating pharma a 5, 6, or 7 on a 7-point scale (7 being very good, 1 being very bad).
Pharma’s focus on science and research as opposed to business has helped take the bad taste from consumer’s mouths regarding price hikes and shady dealings. Big pharma companies that were previously pinned for putting profits over patients are now sharing data and research, helping each other manufacture vaccines and make them accessible, and ensuring their vaccines maintain a high safety standard.
The public is also much more interested in the research and technical details regarding the COVID vaccines and treatments that pharma companies are offering. Thanks to this, brands can not only confidently showcase their vaccine work, but interest a larger audience with other research and innovations. Since consumers are becoming more educated and engaged when it comes to the science behind their health, companies may have an easier time communicating about things such as mRNA, clinical trials, or gene therapy.
Future messages from pharma companies won’t just be about scientific facts though. Eli Lilly CEO Dave Ricks said last April that the biopharmaceutical industry has a “once-in-a-generation opportunity to reset’’ its reputation. The industry is certain to remind the public about its positive COVID-19 performance and heroism through direct-to-patient communications.
Med Device Department: First 3D-Printed Implant for the Talus Bone Approved
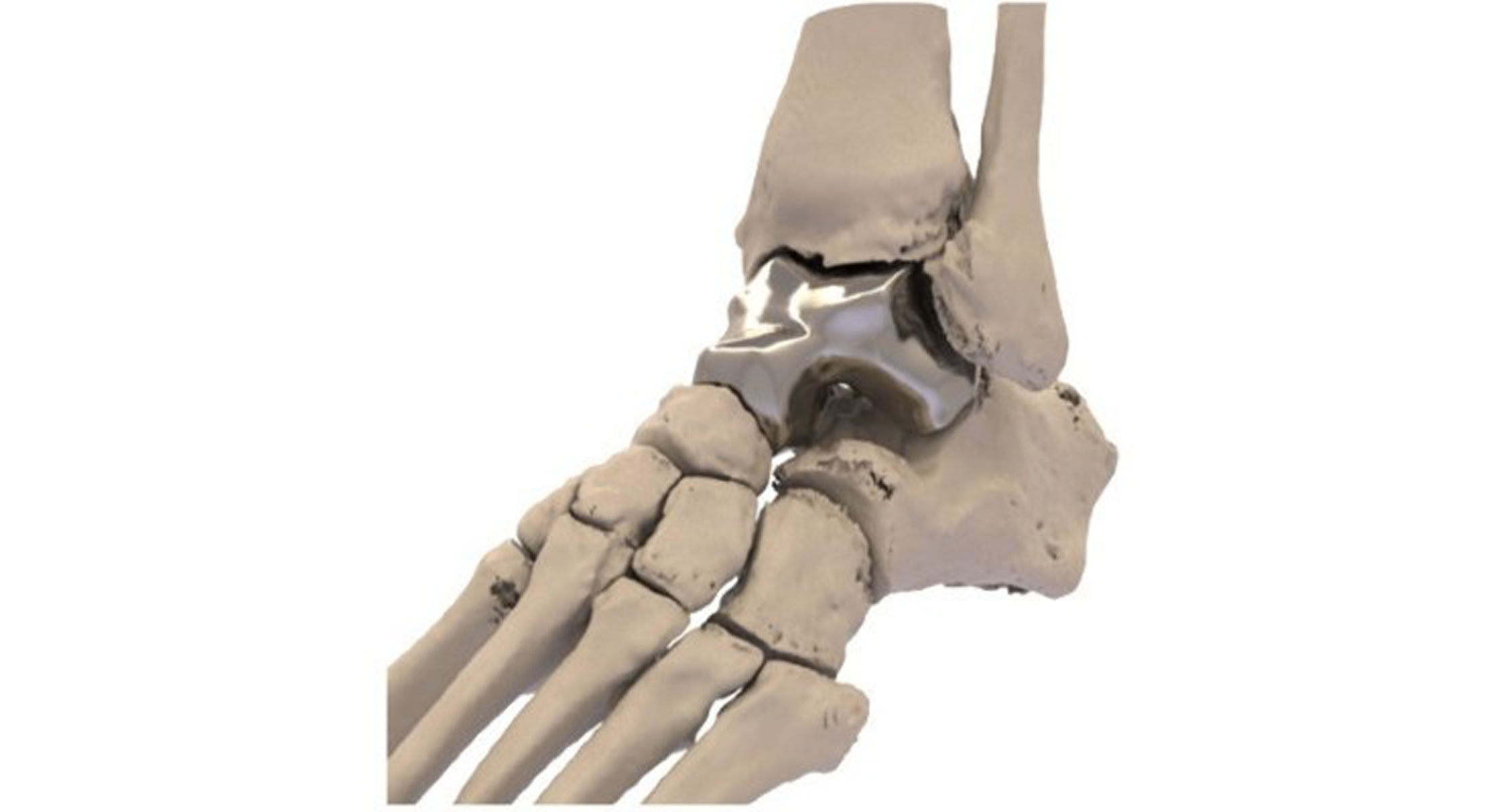
The FDA approved the first-ever 3D-printed implant to replace the main bone in the ankle joint, known as the talus bone, which connects the foot to the leg. The Patient Specific Talus Spacer implant is meant to treat avascular necrosis of the bone, which is a progressive condition that can lead to the death of bone tissue following a sudden injury that cuts off blood flow, such as a broken bone or dislocation.
Normally, the treatment for this injury is drastic, requiring surgical fusion of the ankle and foot bone, which fixes the ankle joint in one position. The implant can help avoid this type of surgery and keep the ankle joint mobile. The implant, made of cobalt-chromium alloy, is 3D-printed according to a patient’s CT scan, ensuring a custom fit.
“Avascular necrosis of the ankle, while a rare condition, is a serious and potentially debilitating one that causes pain and can lead to inhibited motion of the ankle joint, and in some cases, removal of part of the leg,” stated Capt. Raquel Peat, PhD, of the U.S. Public Health Service and Director of the FDA’s Orthopedic Device Office.
New Jersey-based Additive Orthopaedics received a humanitarian use exemption, offered for devices that treat conditions affecting less than 8,000 people annually.
FDA Approvals
Drug Approvals
Juno Therapeutics’ Breyanzi received FDA approval for treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy. Breyanzi is a Car T cell immunotherapy that genetically modifies patients’ T cells to target and eliminate CD19-expressing normal and malignant cells.
Cosela is the first therapy of its class to be approved by the FDA to treat chemotherapy-induced bone marrow suppression in adults receiving certain types of chemotherapy for extensive-stage (when the cancer has spread beyond the lungs) small cell lung cancer. G1 Therapeutics, Inc. developed the drug to inhibit an enzyme, protecting bone marrow from damage during chemotherapy, which helps fight fatigue, infection, bleeding, and other comorbidities.
The Amondys 45 (casimersen) injection from Sarepta Therapeutics was approved for the treatment of Duchenne muscular dystrophy (DMD) in patients who have a confirmed mutation of the DMD gene that is amenable to exon 45 skipping. This is the first FDA-approved targeted treatment for patients with this type of mutation.
Med Device Approvals
The FDA granted emergency authorization to Visby Medical for a rapid, single-use, at-home test for COVID-19. The device plugs into a power outlet to mix a patient’s swab sample with fluids. The fluid is analyzed by the handheld device and delivers a result in about 30 minutes. This authorization allows the test to be used outside of the lab setting, where it has been used since September 2020. Visby miniaturized PCR technology to create a molecular COVID-19 detector that is accessible as rapid antigen tests, which are much less sensitive. The company is in the process of making the device available OTC.
Simplify Medical, Inc. got the greenlight from the FDA for the Simplify Cervical Artificial Disc. This implant can replace the buffering discs between two vertebrae in the neck portion of the spine. The vertebrae bones above and below the implant will grow into the two titanium endplates while the joint remains mobile around a ceramic core. The disc is meant to replace diseased or damaged cervical discs that must be removed, which might otherwise require a spinal fusion. Fusing two vertebrae together does not allow the joint to move, whereas those with the implant can maintain more range of motion after surgery.