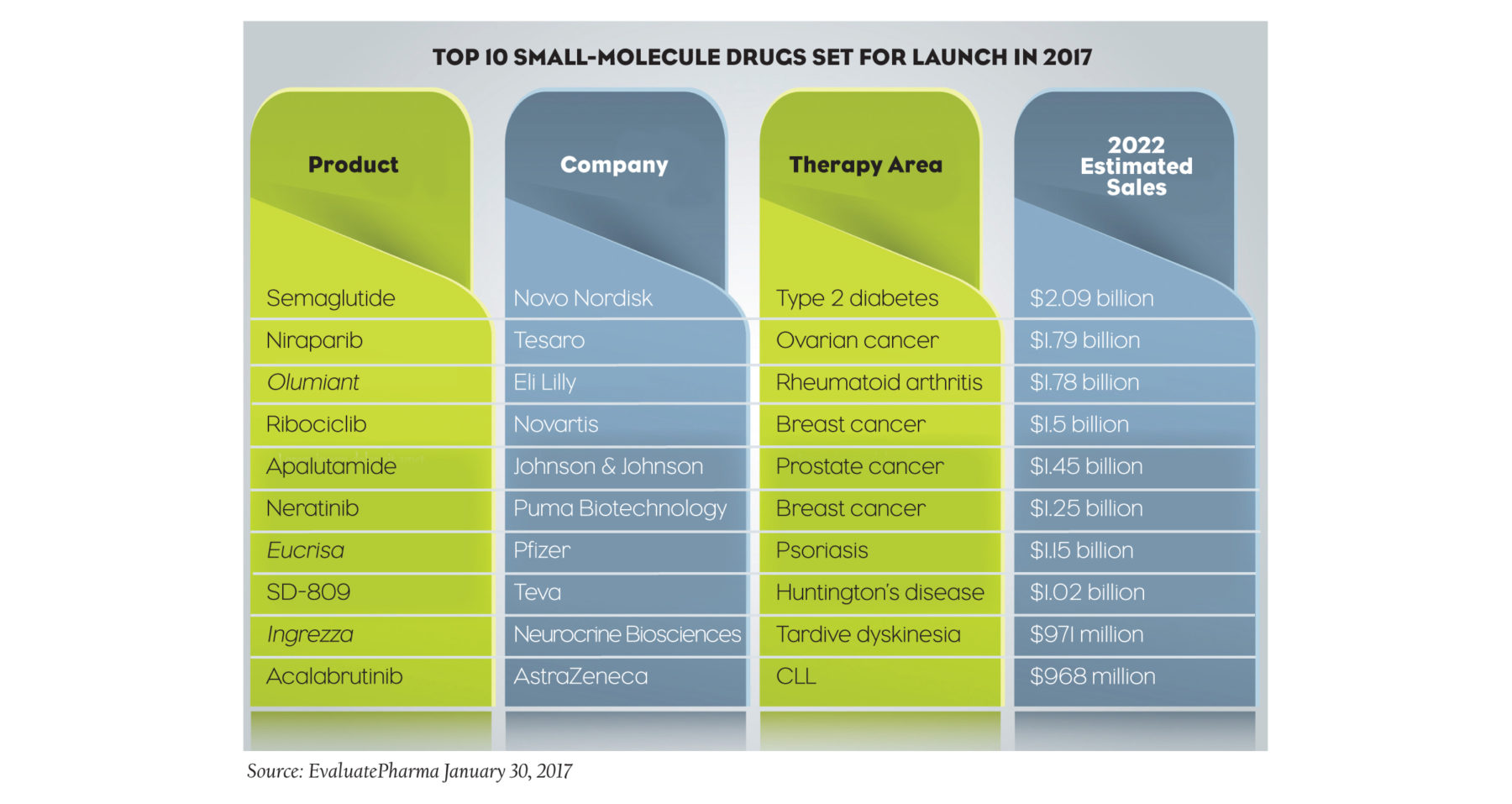
Sales Sector: Estimated Top Selling Drugs Slated to Launch in 2017
Novo Nordisk’s once-weekly type 2 diabetes treatment semaglutide, is expected to be the most successful small-molecule drug launched in 2017, according to EvaluatePharma, a market intelligence firm that forecasts prescription drug sales. Semaglutide is predicted to reach $2 billion in sales by 2022 with the expectation that it will also earn approval for an indication for cardiovascular diseases. The data, which is compiled using sellside consensus 2022 sales forecasts, shows that most of 2017’s biggest launches will be treatments for cancer therapies and autoimmune diseases (see table). Overall, it is expected that 2017 will introduce no fewer than 15 blockbusters to the market.
 [insert Sales Sector image here]
[insert Sales Sector image here]
Discoveries/Innovations: Blood-repellent Materials Could Lead to Better Medical Devices
Over time, medical implants such as stents, catheters, and tubing, can cause undesirable risks in patients such as clots or obstructions that can lead to heart failure or embolisms. But engineers at Colorado State University are offering one possible solution: Materials extremely repellent to blood.
Arun Kota, Assistant Professor of Mechanical Engineering and Biomedical Engineering, and Ketul Popat, Associate Professor in the same departments, joined together to create a specially grown, “superhemophobic” titanium surface that can trick blood into believing that a device contains virtually no foreign material.
This may seem counterintuitive as biomedical scientists typically use materials “philic” (with affinity) to blood to make them biologically compatible. But as Kota explains, “We are taking a material that blood hates to come in contact with, in order to make it compatible with blood.”
Further test are still required, but Popat adds, “If we can design materials where blood barely contacts the surface, there is virtually no chance of clotting.”
Therapeutic Talk: Soft Robotics May Lead to New Treatment of Heart Failure
While devices are currently available that can help augment cardiovascular functions weakened by heart failure, they typically require patients to also take blood thinner medications to reduce the risk of clotting. But researchers at Harvard University and Boston Children’s Hospital have developed a customizable soft robot that fits around a heart and helps it beat, without coming into contact with blood.
This thin silicone sleeve uses soft pneumatic actuators placed around the heart to twist and compress the sleeve in a similar motion to the beating heart. The sleeve is attached to the heart using a combination of a suction device, sutures, and a gel interface. In a paper published in Science Translational Medicine, the researchers explain that the sleeve is also customizable to each patient as the actuators can be tuned to give more assistance to whichever side of the heart is weakest for that particular patient. Furthermore, the pressure of the actuators can also increase or decrease over time, as the patient’s condition evolves.
“This work represents an exciting proof of concept result for this soft robot, demonstrating that it can safely interact with soft tissue and lead to improvements in cardiac function,” says Conor Walsh, senior author of the paper and the John L. Loeb Associate Professor of Engineering and Applied Sciences at SEAS and Core Faculty Member at the Wyss Institute. “We envision many other future applications where such devices can delivery mechanotherapy both inside and outside of the body.”
Medical Devices: New Robot Leads to a World’s First Surgery
Until now, it was thought to be impossible—or just highly improbable—to safely dissolve a blood clot from the retinal vein in patients suffering from retinal vein occlusion, a disorder that can lead to blindness. After all, a retinal vein is only 0.1 millimeter wide—similar to a human hair—and any surgeon would need to be able to inject a needle directly into it and hold steady for 10 minutes or risk damaging the vein or retina.
But after seven years of research, a collaboration between KU Leuven engineers and University Hospitals Leuven ophthalmologists has proven that it is in fact possible—with a little help from a newly designed surgical robot. Unlike most surgical robots, there is no need for a joystick to operate the device. And in addition to the robot, the team also found a way to produce an ultrathin injection needle that is barely 0.03 millimeters wide. The surgeon simply guides the needle into the vein while the robot eliminates any vibration of the needle—increasing the level of precision more than tenfold. After locking the robot, the needle and the eye are automatically stabilized and the surgeon can then inject a thrombolytic drug into the vein to dissolve the blood clot.
The surgery was successfully completed on January 12, 2017 for the first time on a University Hospitals Leuven patient. The patient is doing well and can now start working on the rehabilitation of the eye. Meanwhile, physicians will continue to study the clinical effects of the procedure.
Doctor Docs: Can Artificial Intelligence Replace a Doctor’s Visit?
 Not everyone has time to visit a doctor, or has access to one, to get something like a mole checked out. But soon, you may simply be able to scan it with your phone to get a diagnosis. Computer scientists at Stanford developed an artificially intelligent diagnosis algorithm for skin cancer that matched the performance of 21 board-certified dermatologists, according to a paper published in Nature.
Not everyone has time to visit a doctor, or has access to one, to get something like a mole checked out. But soon, you may simply be able to scan it with your phone to get a diagnosis. Computer scientists at Stanford developed an artificially intelligent diagnosis algorithm for skin cancer that matched the performance of 21 board-certified dermatologists, according to a paper published in Nature.
To achieve this, they compiled a database of nearly 130,000 skin disease images representing more than 2,000 diseases. Then, instead of building an algorithm from scratch, they used one developed by Google that could identify 1.28 million images from 1,000 object categories. The researchers used the images they compiled to teach it to distinguish between different skin cancers.
The algorithm was then tested against 21 dermatologists who were shown more than 370 high-quality, biopsy-confirmed images and asked, based on the image, if they would proceed with biopsy or treatment, or reassure the patient. The algorithm matched the performance of the dermatologists to at least 91% accuracy. Currently, the algorithm only exists on a computer, but the researchers hope to make it available on smartphones in the near future after further testing.
FDA Update
GDUFA Reauthorization
The FDA is seeking information and public comment, in anticipation of the passage of Generic Drug User Fee Amendments reauthorization (GDUFA II), which will impact FDA’s planned approach for administering generic drug program fees for the 2018 fiscal year. Comments can be made at http://bit.ly/2kyvWxH until March 10.
Drug Approvals
Bristol-Myers Squibb’s Opdivo (nivolumab) was approved for the treatment of patients with locally advanced or metastatic urothelial carcinoma whose disease has progressed during a period of up to one year after first-line platinum-containing chemotherapy.
Trulance (plecanatide) from Synergy Pharmaceuticals was approved for the treatment of Chronic Idiopathic Constipation (CIC) in adult patients.
Med Device Approvals
St. Jude Medical, now a part of Abbott Laboratories, got approval of its Assurity MRI pacemaker and the related Tendril MRI insulated lead that delivers current to the heart tissue. The Assurity MRI is the world’s smallest “wireless” MRI-compatible pacemaker. Non-MRI-approved pacemakers carry a low risk of heating up inside the body or experiencing a programming malfunction.
Gore received approval for its Viabahn VBX endoprosthesis to be used in the iliac arteries, making it the only balloon expandable stent graft having such an indication.
Arterys’ Cardio DL became the first technology to be cleared by the FDA that leverages cloud computing and deep learning in a clinical setting. The Cardio DL provides automated, editable ventricle segmentations based on conventional cardiac MRI images that are as accurate as segmentations performed manually by experienced physicians.






