Thanks to the Pfizer/BioNTech and Moderna COVID-19 vaccines, it is likely you have heard the term mRNA. However, it is unlikely you have heard it in the context in which Anima Biotech is exploring this space. Anima is developing Translation Control Therapeutics, which takes a novel approach to discover small molecules that can selectively control mRNA translation in order to treat diseases with previously undruggable proteins or hard targets.
PM360 spoke with Co-founder & CEO Yochi Slonim about how Anima is literally lighting the way to new drug discoveries, the exploding mRNA space, and why they are eager for as many big pharma partners as possible.
PM360: Why did you found the company? How did it get its start?
Yochi Slonim: It’s actually a very good question with regards to Anima because it highlights the very nontraditional path the company has been taking from the beginning and up until now. Anima was founded as an academic project, which is not unusual but what was interesting is that right from the beginning there was a company.
We were living in the biochemistry labs of the University of Pennsylvania (UPenn) based on technology that was licensed from the university. The company was essentially funded throughout the research phase and then afterward, only by private investors. Anima doesn’t have any venture capital funds in the company.
Initially, only $20 million came into the company from private investors and the company has been relying on partnerships with pharma mainly to grow and maintain the operation. For example, we’ve been extremely successful with our research collaboration with Lilly in which they provided $30 million upfront and $14 million in research funding. Since then, our collaboration has grown and expanded. The backing of our private investors and our collaboration-focused approach has allowed the company to grow free from the pressure of venture capitalists and to drive our own agenda.
Anima’s work revolves around mRNA translation. Obviously, mRNA has gotten more attention recently due to the COVID vaccines, but can you explain what kind of work you are actually doing within the space?
Indeed, almost every person in the world today knows the word “mRNA” because of the COVID-19 vaccines. Those vaccines are designed around the technology that is injecting synthetic mRNA that mimics the viral protein and triggers the immune system to create a response. But these are vaccines, not drugs that treat non-infectious diseases like cancer for example.
To put things in perspective, targeting mRNAs is actually not a new approach. It has been considered a very promising area for over 30 years. But until quite recently it was just an idea in the unproven and unvalidated stage. Just two years ago, the first drug that was targeting mRNA came to the market. That technology is known as RNAi or RNA interference.
Traditional drugs are designed to try to go after the proteins causing a disease, especially the small molecule drugs. They do that by binding to a pocket on the protein where the active chemistry of the protein is happening. The molecule goes inside that pocket and “neutralizes” the activity of the protein. But because not all proteins have a pocket that molecules can bind to, a different approach was needed. What if you could possibly “knock down” the mRNA? That way, you would be basically preventing the proteins from actually being made in the first place. They did this, but those drugs have one big drawback: they are not small molecules. They cannot be taken as oral pills and have to be injected. They also have many other “delivery” problems that continue to be a major challenge.
So about three years ago, the industry started looking into how to apply this approach to small molecule drugs. Anima is in that space of targeting the biology around mRNA with small molecules in order to develop drugs that have not been available before against diseases that today are completely untreatable by conventional small molecule drugs. This is a very promising area because if you could do this with small molecules, those drugs could become available on a massive scale.
What else makes your approach to mRNA unique compared to the others in this space?
Most of the other companies in the small molecule space are trying to come up with small molecules that directly bind to the mRNA. Essentially, the mRNA is their “target.” However, it is still an unvalidated approach in small molecules because such binding is not a “knock down” in that sense and it can have many unclear side effects.
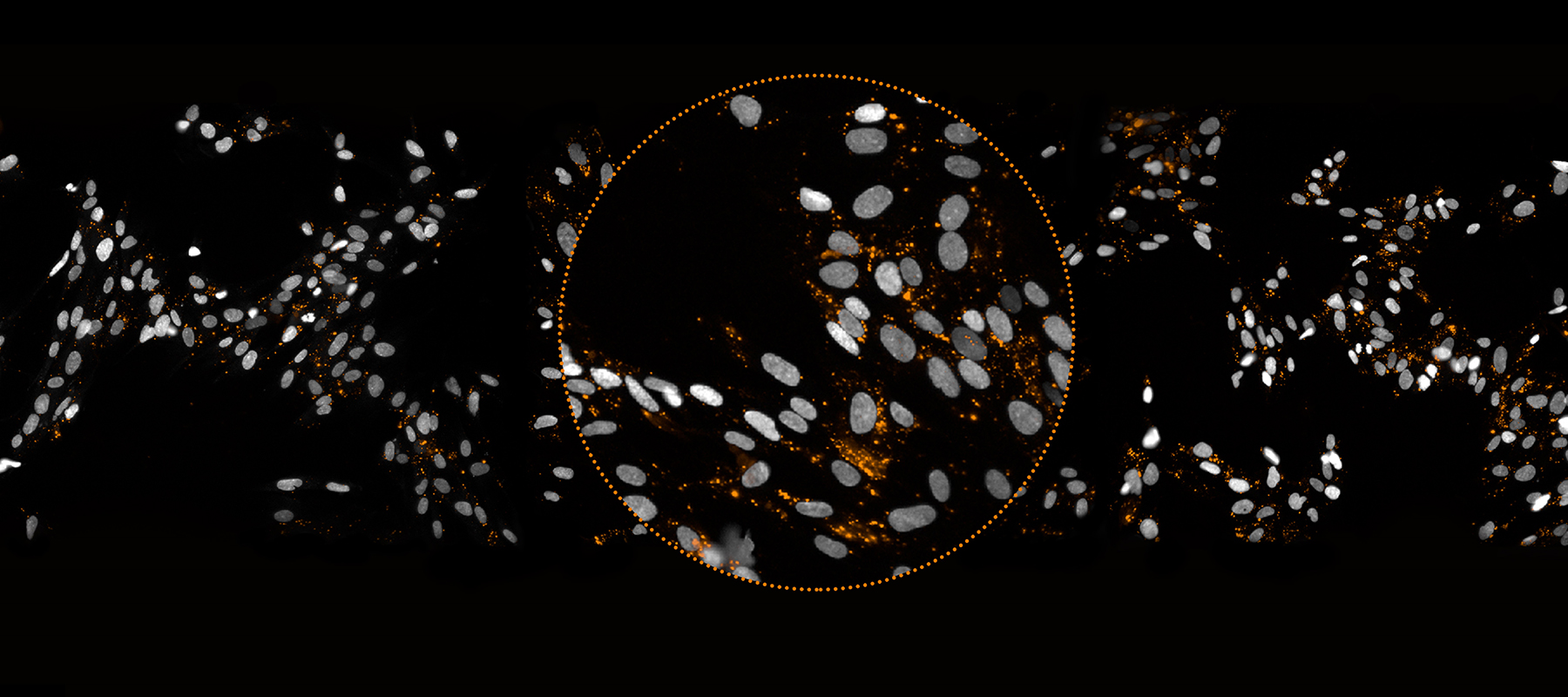
Anima took a very different approach right from the beginning. There are cellular machines called ribosomes in cells. What the ribosomes do is go over the mRNA and according to the code in the mRNA sequence they build the chain of amino acids that makes the protein, and then the proteins fold into its functional final form. We had a crazy idea, which was that we could visualize the process by which proteins are made—visualize it with light pulses emitted from the ribosomes as they go along the mRNA.
Using our technology, we insert into the cells a pair of tRNAs that are labeled with fluorescent colors, red and green. Then the magic happens through an effect called “FRET” whenever such a pair ends up sitting in a ribosome. You then start seeing light coming out of the cells. For example, in the image below from our lung fibrosis program the light dots that you see are exactly the places in those cells where the ribosomes are making—in real time—the protein associated with the disease, which in this case is collagen type 1.
 This provides a completely different level of understanding of the disease—essentially, you are “seeing the disease happening” while everyone else can see what happened “after the disease.” This allows us to screen for drugs that could control the production of that protein. As the protein is being made, all cells are broadcasting light, but in each “well” of cells we are trying a different molecule in it. There are hundreds of thousands of such “disease samples” and the whole process runs completely automated in our screening system.
This provides a completely different level of understanding of the disease—essentially, you are “seeing the disease happening” while everyone else can see what happened “after the disease.” This allows us to screen for drugs that could control the production of that protein. As the protein is being made, all cells are broadcasting light, but in each “well” of cells we are trying a different molecule in it. There are hundreds of thousands of such “disease samples” and the whole process runs completely automated in our screening system.
Some of these molecules—a very small number of them—may affect the light. If they do, it is because they interfere with the biology through which the ribosomes are making the proteins. If they reduced the light, it means that they inhibiting the translation or reducing the making of proteins by ribosomes of that protein. And if they increase the light, it is because they are increasing the translation. These molecules are “hits,” they are actively controlling the production of the protein so they are possibly the beginning point of a new drug to treat the disease.
Back to the lung fibrosis example. In the image below on the left (activated lung fibroblasts), you can see this cloud of light on the top right corner, which is the disease happening inside the lung as all of this collagen is being made. Given that the collagen is a completely undruggable target, there is no way to bind to that protein. Even if you could, that would be a very bad strategy because the last thing that you would want is to kill all the collagen. You want a very selective way to stop the production of this collagen and only in the lungs, where the disease is located.
Then in the image in the middle (hit compound – decrease in collagen 1 translation), that is exactly what these drugs are doing. These compounds have almost eliminated the light, returning it back to the healthy state. In a very interesting way, this is working in completely symmetric way—you could even increase collagen if you want (the image on the far right).
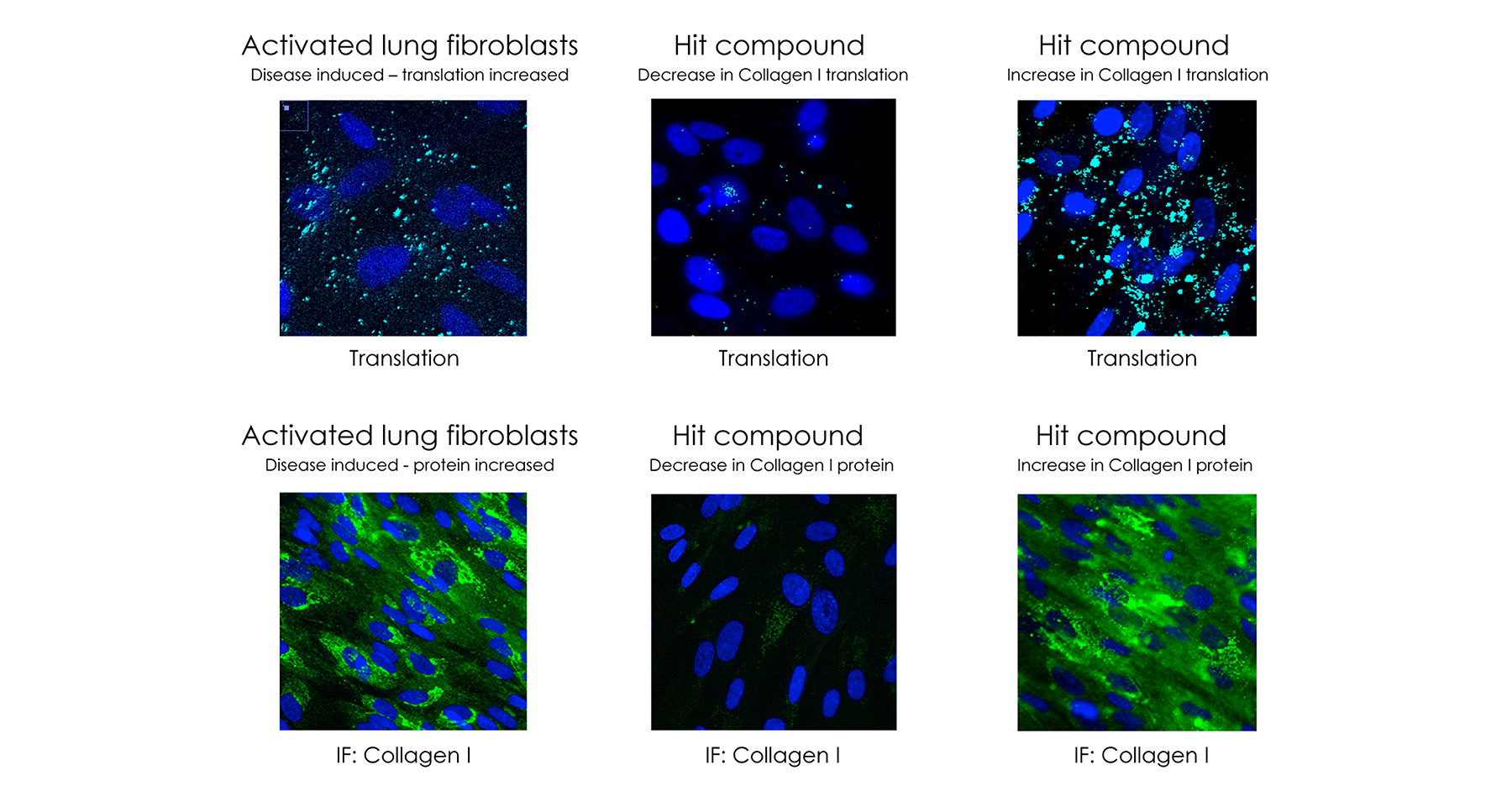 What is amazing about this approach when compared to direct targeting of mRNAs, is that when you target the mRNA directly, you cannot hope to have any tissue selectivity. Wherever and whenever that mRNA exists in the body, the drugs will hit it, which can cause systemic side effects. This is a major concern for small molecule drugs that target mRNAs and in general “selectivity” is the main issue here. However, our approach is not going after the mRNA, but the proteins around the mRNA that are regulators of protein translation. You can actually find tissue-selective drugs that work—like you saw above—only in the lungs. They don’t touch the collagen in the liver, skin, kidney, etc. They are small molecules of a new phenomenon. It’s like a “drug that finds the disease.”
What is amazing about this approach when compared to direct targeting of mRNAs, is that when you target the mRNA directly, you cannot hope to have any tissue selectivity. Wherever and whenever that mRNA exists in the body, the drugs will hit it, which can cause systemic side effects. This is a major concern for small molecule drugs that target mRNAs and in general “selectivity” is the main issue here. However, our approach is not going after the mRNA, but the proteins around the mRNA that are regulators of protein translation. You can actually find tissue-selective drugs that work—like you saw above—only in the lungs. They don’t touch the collagen in the liver, skin, kidney, etc. They are small molecules of a new phenomenon. It’s like a “drug that finds the disease.”
What kind of results are you seeing from the drugs you are developing?
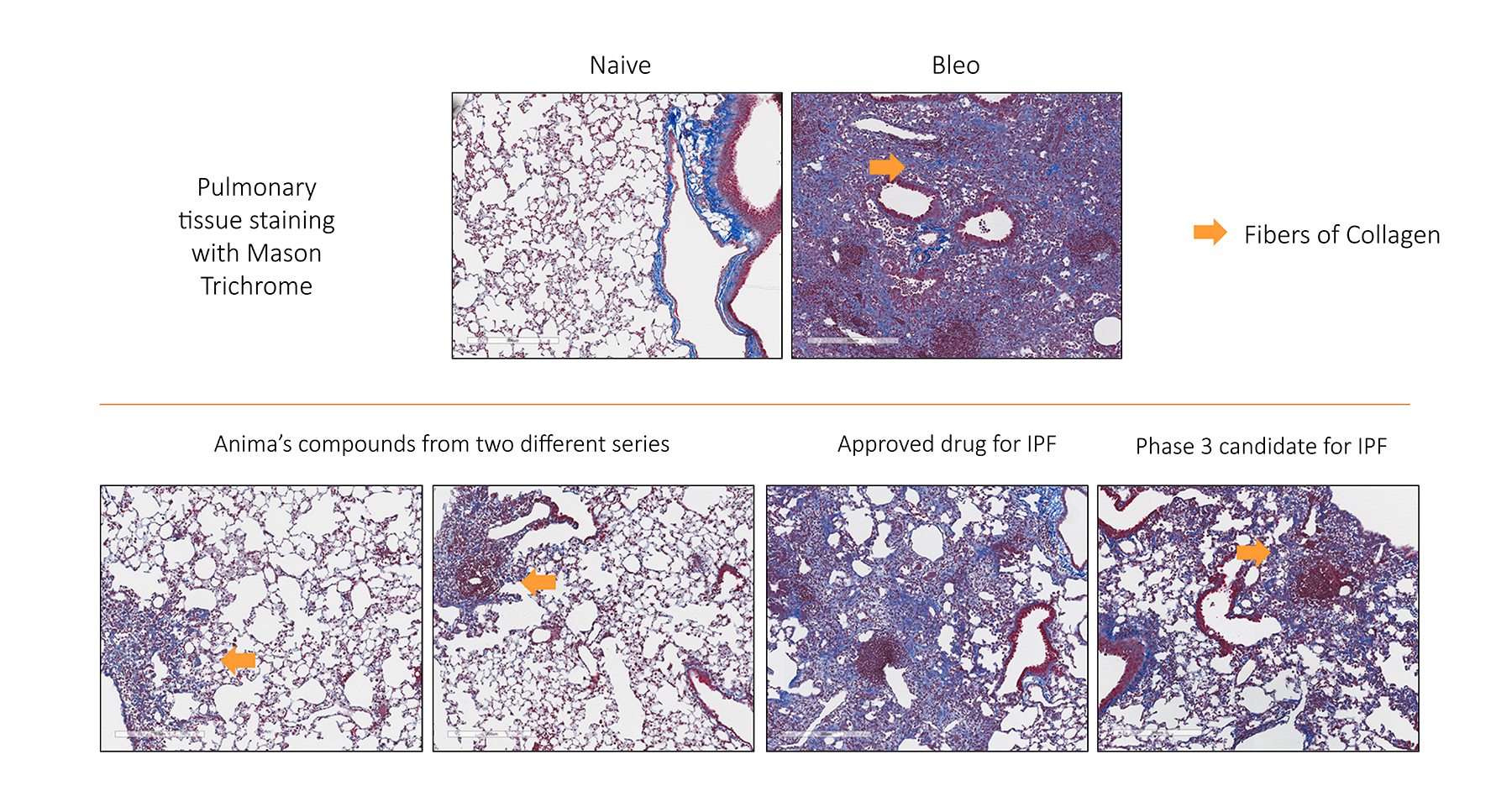
Our most advanced program is the one for lung fibrosis, which is now about 10 months away from the clinic. So it’s still preclinical, but we’ve demonstrated in animal studies the superiority of those drugs. In the image below we compare our molecules with two existing ones on the market. You can see that the two competitors are not really working that well since can a lot of collagen remained in the lung. But with the compound from Anima, there is complete elimination.
 In addition to lung fibrosis, we have a program in oncology with c-Myc, which is one of the most famous undruggable proteins of all times and is known as being over-expressed in about 70% of the cancer. We identified selective c-Myc translation inhibitors and identified their mechanism of action and molecular targets. We also have an oncology program in K-Ras, another very famous hard target in cancer. Then there are our programs in neuroscience for Huntington’s disease, a strategy against other repeat associated diseases, and finally a program in infectious diseases for respiratory syncytial virus (RSV).
In addition to lung fibrosis, we have a program in oncology with c-Myc, which is one of the most famous undruggable proteins of all times and is known as being over-expressed in about 70% of the cancer. We identified selective c-Myc translation inhibitors and identified their mechanism of action and molecular targets. We also have an oncology program in K-Ras, another very famous hard target in cancer. Then there are our programs in neuroscience for Huntington’s disease, a strategy against other repeat associated diseases, and finally a program in infectious diseases for respiratory syncytial virus (RSV).
How did you choose those areas to focus on?
We can actually find small molecules that selectively control the production of 80% of the human protein individually, so the number of potential targets to work on is just enormous—around 16,000. To start, we chose the major areas where there is a potential for expansion and an undruggable or a very hard target. For example, in oncology, we created a shortlist of 100 hard targets that the whole industry is looking at, but very few programs exist to go after them. When we talk to pharma partners in the oncology space, we show them the results of the earlier research we did over those targets and the ability to go after them, which can drive a collaboration project around those targets.
Building off that, what do you look for in partnerships with pharma?
For us, partnering is a main strategy, it’s not on the sidelines. We look at our partners as the vehicle to drive into the market. We believe that big pharma is extremely more capable in doing that than we are on our own, especially in small molecules. We are looking to partner as early as possible across the broadest range of indications.
Looking more long-term, what do you hope this company accomplishes five to 10 years from now?
The trajectory of Anima is really a derivative of the trajectory of the whole space of mRNA. mRNA is an extremely promising space for new drugs. It’s a completely different idea to find drugs that work “before the disease happens.” I would say over the next five to 10 years, as those drugs will get to the clinic—if there are positive results—this will be explosive. Our vision is to discover drugs to fight hundreds of diseases. The only way to do that is to partner with as many pharma companies as you can. So, 10 years from now, I hope I can say we have 200 programs partnered with pharma with 40 of them in the clinic. Ultimately, we want to bring this value on a massive scale to the world—to the patients.