Because of memorable direct-to-consumer advertising campaigns, including the “Gotta Go” Detrol campaign that became part of American pop culture, many adults have some familiarity with overactive bladder (OAB) and its treatments. Brands that treat OAB face common issues, including patients’ reluctance to visit their physician, non-drug treatment options, generic competition, and inconsistent efficacy. But even with these challenges, some manufacturers, particularly Pfizer and Astellas, have chosen to extend their presence in the market.
TREATMENTS
Experts believe that OAB is under-diagnosed, although estimates of prevalence vary. Common belief is that patients with OAB, which is characterized by sudden, strong urges to urinate, may be embarrassed to see a physician about their condition or believe that its symptoms are a natural result of aging. When patients do visit their physicians, they’re able to discuss a number of treatment options. Prior to prescribing a drug, physicians may first recommend physical or behavioral therapy or lifestyle modifications.
There are a number of OAB medications available, most of which are branded. Oxybutynin chloride and oxybutynin chloride ER, generic versions of Ditropan and Ditropan XL, are the only generics currently competing in the market. However, more generics soon will arrive, as Detrol is expected to lose patent protection later this year.
In the 12 months ending January 2012, more than 17.8 million prescriptions were filled for drugs in the market, accounting for almost $2.1 billion in sales. Astellas’ VESIcare, which was promoted jointly with GlaxoSmithKline, led the market with 3.8 million prescriptions. Oxybutynin chloride and oxybutynin chloride ER followed closely with 3.7 million and 3.6 million prescriptions, respectively. Pfizer’s Detrol LA, which previously led the market, ranked fourth in total prescriptions with 3.3 million. Warner Chilcott’s Enablex and Pfizer’s Toviaz followed with 1.6 million and approximately 712,000 prescriptions, respectively.
Market share for VESIcare and the two generics has been increasing and will continue to do so because they are the drugs most often prescribed to new-to-market patients and those switching treatments. This dynamic market segment, which represented 15% of prescriptions in January 2012, is a brand’s greatest growth opportunity (figure 1). Among new patient prescriptions, oxybutynin and VESIcare led with 27% and 24%, respectively.
PFIZER’S SHIFT
VESIcare, however, hasn’t always been the leading brand. As recently as April 2011, Detrol LA led the market in total prescriptions (figure 2). Detrol LA has dominated the market for years, but the brand’s prescriptions began to decline in mid-2009 and continued to decrease thereafter. So what happened in 2009 that changed Detrol LA’s trajectory? Pfizer launched Toviaz and shifted all promotion away from Detrol LA in favor of the new brand. Toviaz, which became available in March 2009, isn’t considered a true line extension but is structurally similar to Detrol and Detrol LA.
Pfizer’s promotional shift from supporting Detrol LA to Toviaz wasn’t novel. In fact, many companies have done the same when launching a line extension. It wouldn’t even be worth highlighting had Toviaz grown rapidly and replaced much or all of Detrol LA’s original share of the market. But Toviaz’s monthly market share has not come close to mimicking Detrol LA’s success. In January 2012, Toviaz accounted for only 4% of prescriptions in the market.
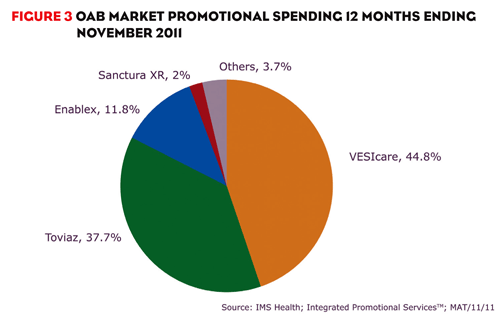
Although Toviaz’s performance has been questionable, Pfizer hasn’t given up. At the end of 2011, Pfizer reported positive top-line results of a recent study for Toviaz and continues to promote the drug heavily. In the 12 months ending November 2011, over $252 million was spent promoting the brand, accounting for 38% of promotional spending in the market (figure 3).
ASTELLAS’ FUTURE
VESIcare is currently the leading OAB brand and isn’t expected to lose patent protection until 2018. VESIcare has been supported with significant promotion, accounting for 45% of promotional spending in the OAB market during the 12 months ending November 2011. VESIcare has been promoted by a much larger field force than competitors, but that may change as GlaxoSmithKline is no longer marketing the brand.
Moving forward, VESIcare might not be the only OAB product offered by Astellas. In August 2011, Astellas submitted a New Drug Application to the U.S. Food & Drug Administration for mirabegron. If approved, mirabegron will be a first in class treatment for OAB—one with a different mode of action than the currently available medications.
With two drugs in the same market, Astellas will face a situation similar to what Pfizer encountered in 2009, although with some significant differences. VESIcare is the current leader among brands, but its dominance is not nearly as significant as Detrol LA’s was when Toviaz was introduced. In addition, as mirabegron will offer a different mode of action than VESIcare, it will not be viewed as a line extension.
Knowing the risk of pulling back promotion, it will be interesting to see how Astellas chooses to handle marketing VESIcare if mirabegron is approved. It may choose, as Pfizer did, to pull back promotion for VESIcare completely so its field force can concentrate on a strong launch. Or, if it has the resources, it may choose to market both. If Astellas does market both, it’ll need to message each in a way that doesn’t negatively affect the other, by possibly positioning them for different patient segments.
The OAB market’s future with the potential launch of mirabegron, introduction of generic Detrol, and Pfizer’s continued investment in Toviaz is sure to be one of much fluctuation.
FIGURE 1:
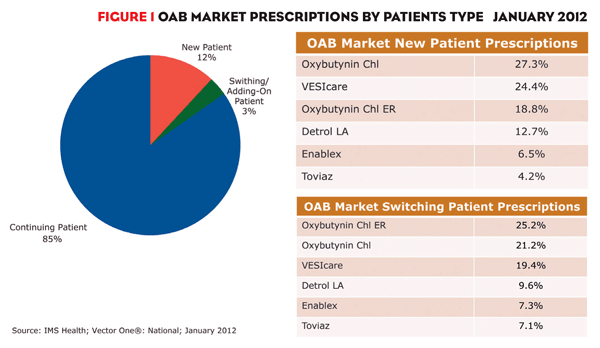
FIGURE 2:
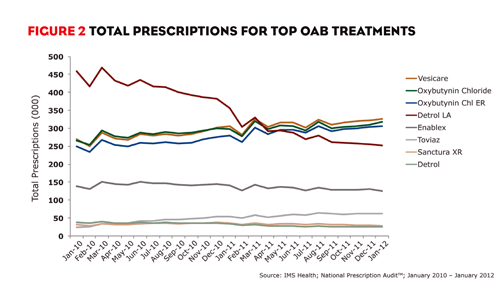
FIGURE 3: