Patients often don’t have time to wait. But, the clinical trial process can be slow. Even in the digital age, getting a trial up and running can get bogged down from manual and archaic processes ingrained in our industry. Kim Walpole, Co-founder and CEO of Trials.ai, decided it was time to speed things up by using artificial intelligence to help design better trial protocols and identify ways to run more efficient trials.
PM360 spoke with Kim about what prompted her to shake up the clinical trial process, how the company’s AI algorithm can make trials better for all involved, and the benefits that trial sponsors will get from following the algorithm’s feedback.

PM360: Why did you start Trials.ai?
Kim Walpole: I spent over 12 years working with pharma companies on the strategic planning side. Then about three years ago, my best friend, Paul, got diagnosed with pancreatic cancer, and it was one of those life-altering moments. I started reaching out to friends to try to get him in a clinical trial, and what I kept hearing was there were a couple of promising therapies—but by the time they got them clinical trial-ready, he would be gone.
Unfortunately, that was the truth. He ended up dying within eight months. Having been in the industry for 12 years, I clearly understood the problems, the logistical challenges, and the archaic manual processes. But until that moment, it was somebody else’s problem to fix. When Paul died, I decided if nobody else is going to do it, then I’m definitely going to do it
How did you go about getting the company off of the ground?
I was able to pull together a small team and we started working on it. Fortunately for us, we also had an academic institution that was looking for ways to accelerate their trials. In fact, some physicians came to us and said, “I heard what you’re wanting to do. We wrote a grant, and we actually have the money to put towards helping you build out this technology.”
How does your technology work to make trials more efficient?
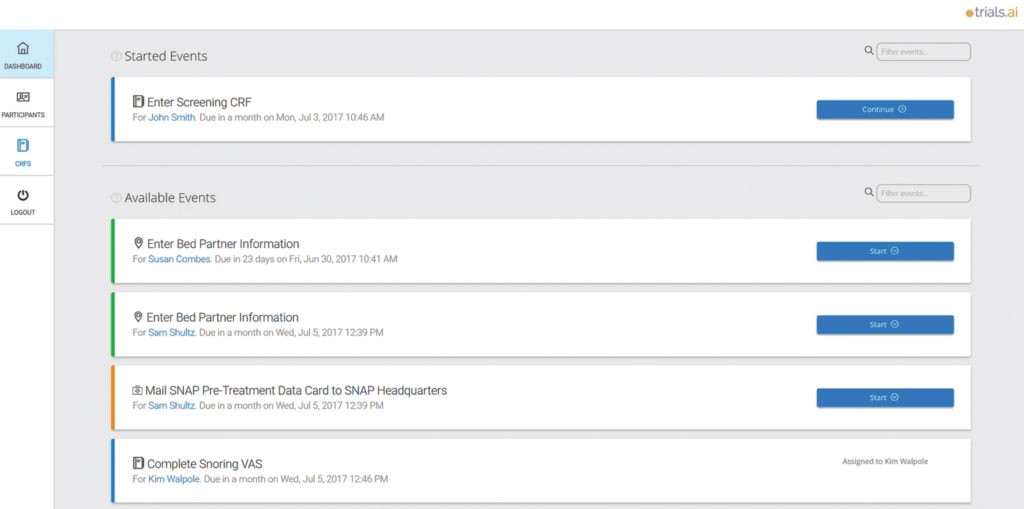
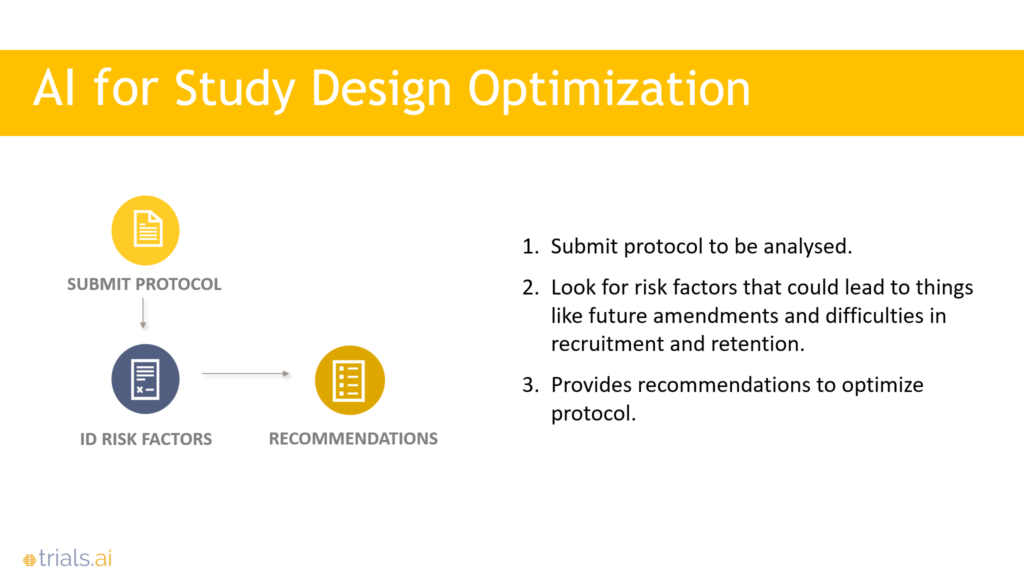
The reality is that therapies, drugs, and devices get stuck in the clinical trials process because of poor protocols and lack of great tools to conduct trials. The industry is still dealing with siloed systems, spreadsheets, and a lack of real-time data. Our focus is to leverage artificial intelligence to help organizations design better protocols, and then, to improve their trial management workflows.
Where is the data coming from to allow the AI to determine better protocols?
Essentially, we are working with three layers of data. Initially, we trained our algorithms with datapoints from publicly available historical data from successful and unsuccessful trials as well as journal articles, research papers, and anything we could essentially get our hands on that was publicly available at that point. That’s the first layer.
Then we also generate our own proprietary data on the workflow side. Meaning, as trials are running we are able to gain not only typical trial data that would go through a DataCast, or electronic data capture system, but also logistical data so that we can understand the interactions between patients as well as sites in real-time. So, that’s the second layer.
For our third layer, we are building into the actual service agreements with trial sponsors that we will be able to use their historic data as well as their real-time data as they’re running trials on our platform, to continue to inform our algorithm. So essentially, the more people that are on the system, the smarter the system gets.
What benefits have companies seen from using the system?
The original academic institution I mentioned reduced their administrative tasks by about three hours per day, and we decreased their data errors by 20%. And that was actually their first trial. Now they’re running a second trial with us, because that was a successful endeavor.
For another customer, we fed their protocols into our algorithms and found some threats around inclusion-exclusion criteria. So, we were able to give them feedback. We have found that a lot of the challenges in recruitment, retention, and data capture stem from problems in the study design or the protocol development. So, that has become our focus. Our goal is to have an interface akin to TurboTax, in which trial sponsors can start with an idea and we can walk them through all of the ancillary pieces of developing that protocol using our algorithm.
You mentioned the system also gives real-time feedback, so can it also provide feedback to improve the ongoing trial as it proceeds?
Yes, and that’s one of the exciting things from the trial sponsor perspective. Traditionally, when they’re running trials, they are relying on consultants or CROs to not only help develop their protocols, but also make sure things are running well at the site level.
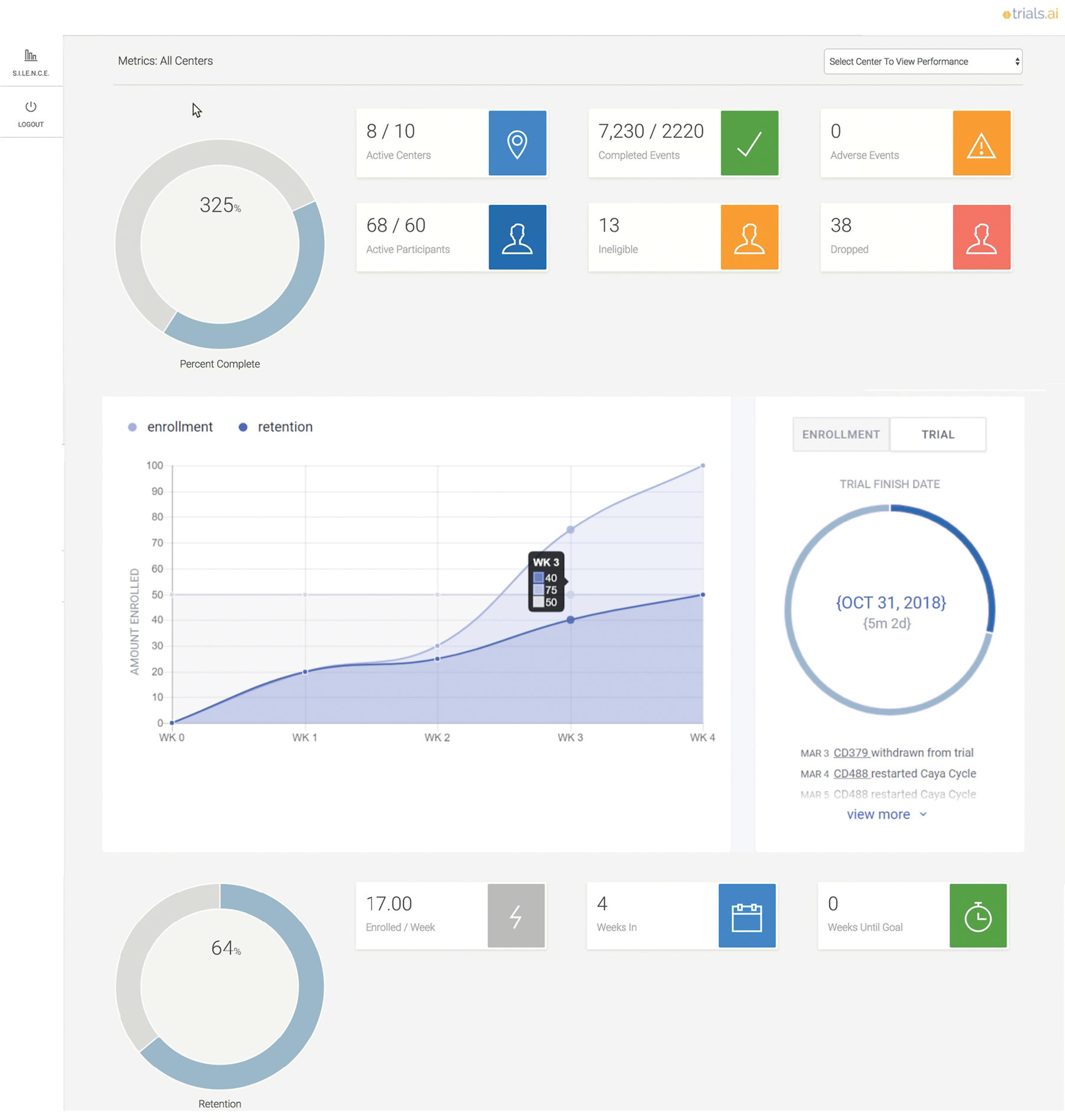
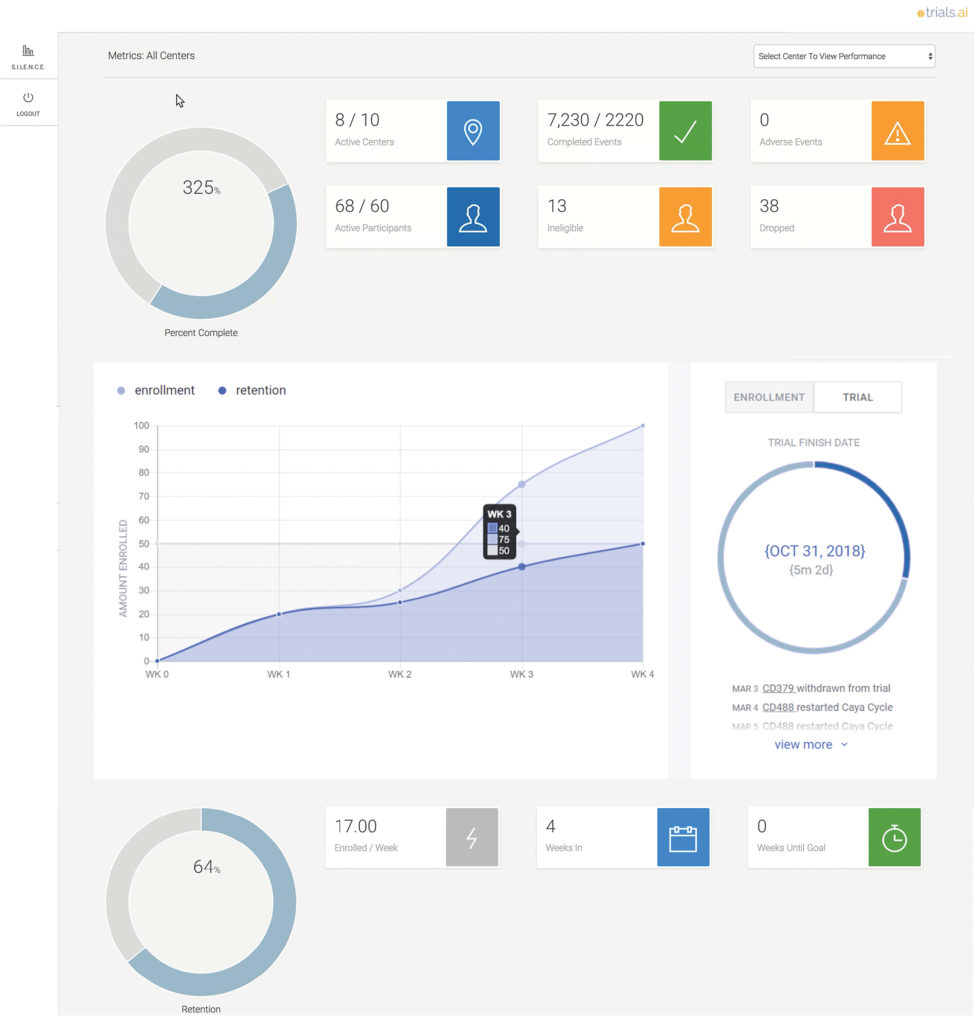
We provide trial sponsors with a very transparent process, so they get their own login and can drill down and see things that are important to them, such as enrollment, retention, serious adverse event information, and data around the site. They get real-time feedback on the health of their studies, and if they see challenges at the site level, they can pick up a phone and have a good, intelligent conversation with the site.
Your company is also doing the “I’m the Why” social media campaign to promote the importance of clinical trials. Why did you want do this campaign?
We started the “I’m the Why” campaign to add faces to clinical trials. Traditionally, the space tends to be pretty sterile, and I started to notice the industry discussion around patient centricity. So, we started this campaign to interact with patients, hear real stories, and hopefully, push researchers as they’re looking for new ways to treat different therapeutic areas. It’s been interesting to step away from the data and to balance that with real stories about real people who are looking for real treatments and have yet to find them. Because ultimately, that’s our mission—to get treatments to patients in need faster.