Should pregnant women or women trying to conceive feel safe about getting the COVID-19 vaccine? It is a question these two groups of women have been asking since the COVID-19 vaccines have become available as they worry about how it may affect their baby or their chances to make one. In fact, it is a question they have asked themselves about most other vaccines as well—and their response has typically been to decide against vaccination.
In an October 2019 Vital Signs report from the CDC, the government agency found that 65% of mothers-to-be in the U.S. had not received two safe and effective vaccines to reduce the risks of influenza (flu) and whooping cough (pertussis). That is despite the fact that pregnant women have more than double the risk of hospitalization compared to nonpregnant women of childbearing age if they get influenza. Because of this, the CDC recommends that pregnant women get a flu vaccine during any trimester of each pregnancy and the whooping cough vaccine during the early part of the third trimester of each pregnancy as part of routine prenatal care. Additionally, the baby also benefits from this as antibodies are passed along to the fetus and protect newborns who are too young to get vaccinated.
Similarly, in April 2021, the CDC released its recommendation for pregnant women to get the COVID-19 vaccine after a study of 35,691 women found “no safety concerns were observed for people vaccinated in the third trimester or safety concerns for their babies,” according to CDC Director Dr. Rochelle Walensky. The researchers of the study, which was published in The New England Journal of Medicine, monitored these women’s self-reported symptoms from Dec. 14 to Feb. 28 using the “v-safe after vaccination health checker” surveillance system, the v-safe pregnancy registry, and the CDC’s Vaccine Adverse Event Reporting System (VAERS). They found that pregnant women reported injection-site pain more frequently than non-pregnant women, but fewer other side effects. Furthermore, the rates of miscarriage were similar to those observed pre-pandemic.
How Do Pregnant Women Feel About the Vaccine?
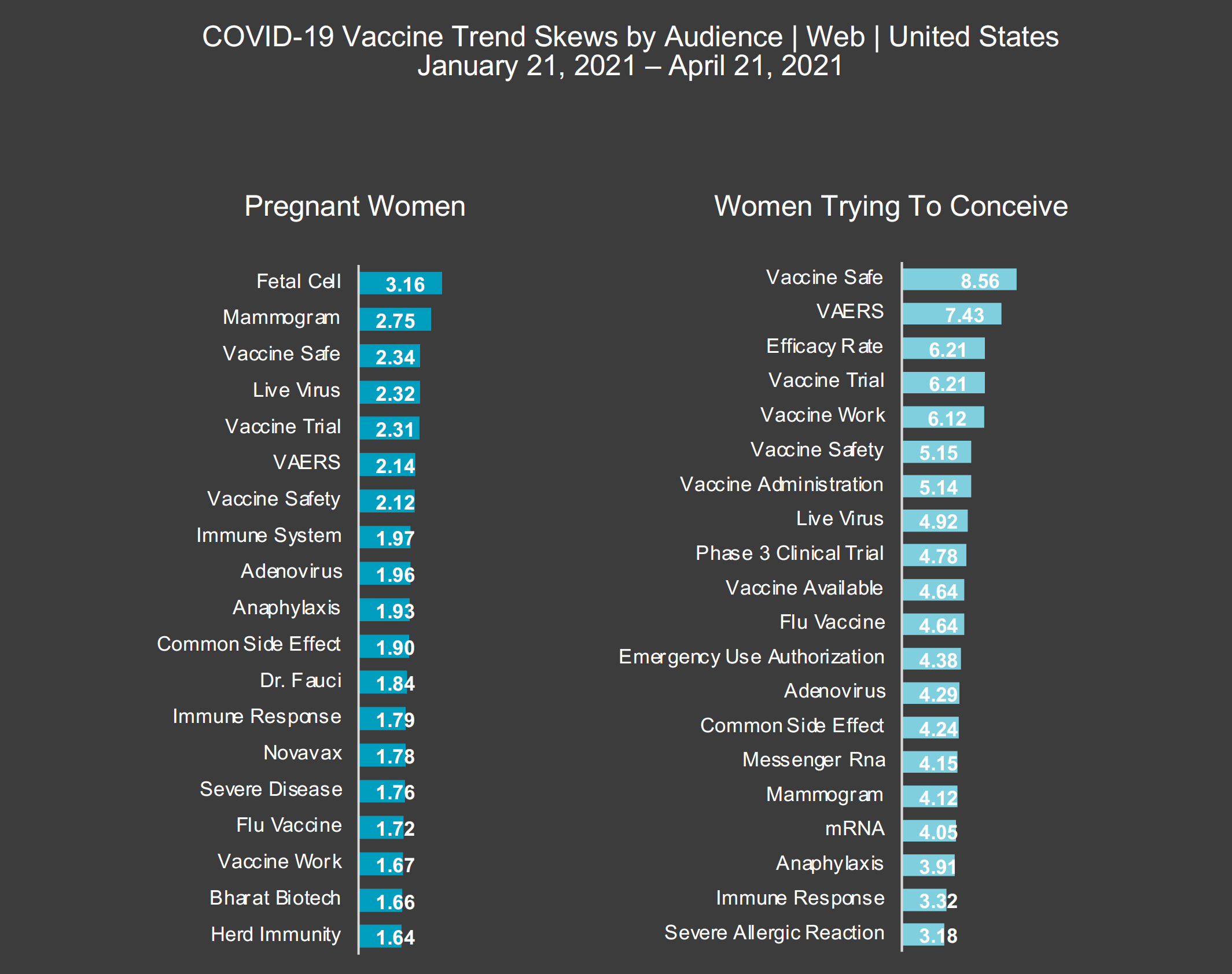
In order to gauge the perception of pregnant women and women trying to conceive about the COVID-19 vaccine, advertising agency platform Amobee tracked their online behavior and engagement regarding COVID-19 vaccine information for three months (Jan. 21, 2021 to April 21, 2021). Overall, women trying to conceive were far more engaged with COVID-19 vaccine trends than pregnant women, something Alex Knudsen, Vice President of Solutions Engineering at Amobee, cites as one of the surprises from their findings when you consider that the perceived notion may be that pregnant women are naturally more concerned for their immune system.
And yet, women trying to conceive were 8.56 times more likely to be engaged with “vaccine safety” than the average person. Comparatively, pregnant women were only 2.34 times more likely to look into “vaccine safety” and were actually most concerned about the use of fetal cells in the development of available vaccines as well as the new mammogram guidelines for vaccinated women.
Knudsen attributes one of the reasons for the increased concern about vaccine safety by women trying to conceive to be a false report that Johns Hopkins Medicine identified as circulating around social media that said the COVID-19 vaccine would affect a women’s fertility.
That could also be why engagement with the CDC’s VAERS by women trying to conceive increased 577% during the three-month period observed as compared to the previous three months. Additionally, their engagement with the “efficacy rates” of the vaccines increased by 109% over that same time frame and their conversations around vaccine safety increased by 88%.
Women trying to conceive were also were more engaged with each of the vaccine brands. While they seemed to prefer Moderna over Pfizer, pregnant women’s interest in the Pfizer and Moderna vaccines were nearly the same. However, both groups of women spent the most time looking into the Johnson & Johnson vaccine.
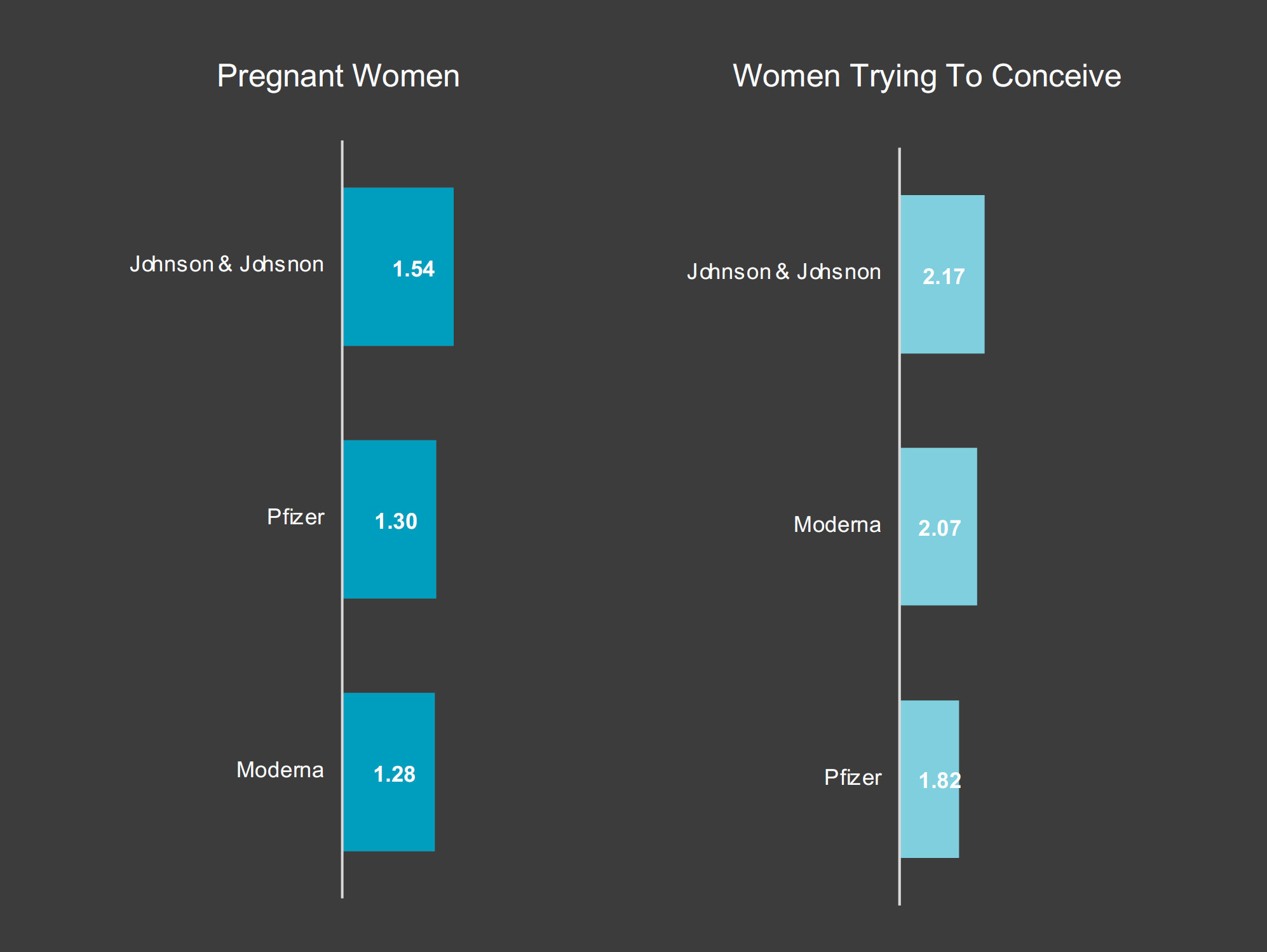
“In reference to Johnson & Johnson, both categories of women were most interested in the content around the vaccine, due to the safety concerns with blood clots,” Knudsen explains. “The top articles consumed by them focused on the CDC and FDA mandated pause. In terms of Pfizer and Moderna, engagement from both groups was preferential as they were seeking additional information about which vaccine to get and where to get it.”
In total, 505.5K women were observed as part of Amobee’s research. As for how pharma marketers can apply these findings to their engagement efforts, Knudsen has some suggestions.
“Pharma marketers should focus their efforts on providing reliable education-based messaging to the pregnant women demographic, especially when it comes to vaccines,” Knudsen explains. “They’ve shown they are susceptible to misinformation and are historically not known to be early adopters of vaccines compared with the general population. Meanwhile, women trying to conceive are likely to be suspicious of vaccines, but are also highly engaged and more likely to conduct extensive research. Marketers should approach these audiences with information and messaging from reputable sources.”








