According to the National Organization for Rare Disorders (NORD), there are over 7,000 known rare diseases, with 90% having no effective treatment method or cure.1 When an individual is diagnosed with a rare disease, they often experience fear, uncertainty, and anxiety. Obtaining a diagnosis is only the first step, leaving many without the direction or resources to find the additional medical care they need.
Clinical trials are an invaluable component of new drug development. Often, these studies are a patient’s only opportunity to receive life-saving or life-changing treatment. Despite this, nearly 80% of clinical trials fail to finish on schedule, with 20% delayed over six months or more.2 While the reasons for these delays vary, an estimated two-thirds of trial sites fail to meet initial patient enrollment target numbers. This problem is especially prevalent with rare and ultra-rare diseases with an extremely small patient population pool.
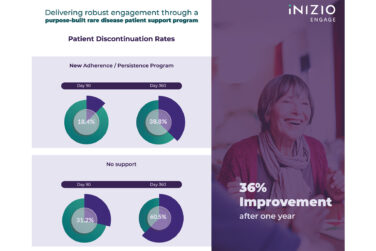
Rare disease clinical trials are notoriously rigorous and offer little flexibility to patients and families. However, contract research organization (CROs) can improve trial efficiency and outcomes by centering the patient experience during the initial trial protocol design.
What Does Patient Centricity Look Like?
As our industry works to develop and define patient centricity in clinical trials, the overarching goal remains—to utilize the most effective tools to improve patient experience and deliver life-saving treatments and life-enhancing products to market sooner.
Understanding what creates a quality patient experience offers trial sponsors and CROs an advantage in today’s competitive drug development landscape. The logistics related to travel are one of the biggest obstacles facing rare disease trial participants. On average, a patient travels 40 miles from home to participate in a study.3 With the limited availability of clinical trial opportunities for rare and ultra-rare disease patients, travel can often be far more extensive, sometimes crossing state and country borders. The additional stress associated with finding local medical care and lodging, securing passports and visas, and obtaining translation and interpretation services often discourages patients and their caregivers from agreeing to this much-needed treatment.
One way these barriers can be combated is through engaging support services in the form of dedicated patient concierges who guide the participant and their caregiver through the entirety of a trial. Investing resources in patient experience programs benefits trial sponsors in several ways, including:
- Improving recruitment, retention, and diversity efforts by reducing barriers hindering patients’ willingness to participate and remain in a trial
- Bringing life-saving and life-enhancing therapies to the market faster by easing day-to-day aspects of the trial, resulting in fewer trial delays
- Reducing the risk of not securing the requisite approval for a drug to reach the market due to poor or inadequate data collection
As the medical community recognizes the immense value in putting the patient experience at the center of clinical trials and ensuring the necessary changes are made, outcomes will improve for both patients and industry stakeholders.
References:
1. https://rarediseases.org/wp-content/uploads/2020/11/NRD-2088-Barriers-30-Yr-Survey-Report_FNL-2.pdf.