The first person to be cured of pancreatic cancer—or the first Alzheimer’s survivor—will likely be a clinical trial participant. Many patients with chronic or terminal conditions could be part of, or benefit from the findings of a clinical trial. It’s why big pharma and biotech companies find themselves in a race to market in many indications. The pressure to enroll patients in clinical trials has never been greater, and never more dependent on sophisticated patient recruitment organizations (PRO).
How are PROs meeting this challenge? With improved data mining, targeted patient outreach, and close attention to metrics. PROs are ensuring that the proper time is devoted to planning prior to project execution to ensure success. Here are some key recommendations to consider.
1. Use patient insights based on data, focus groups, and other audience research.
In order to design the most effective recruitment campaigns, sponsors need to understand the full patient journey and what motivates people to participate in clinical trials. To gain these insights, real-world data is vital. A PRO always should overlay big data, sourced from both in-house databases and external sources, to identify study targets. This ensures the data is robust, and doesn’t rely on a single data point for study-specific targeting.
One of the most effective ways to generate real-world insights into various diseases is patient community building, where the end goal is to develop interest in clinical trials overall. As shown in Figure 1, a PRO may compile a historical patient database of pre-screened trial candidates who understand the benefits of trials, or compile a self-reported health database of households.
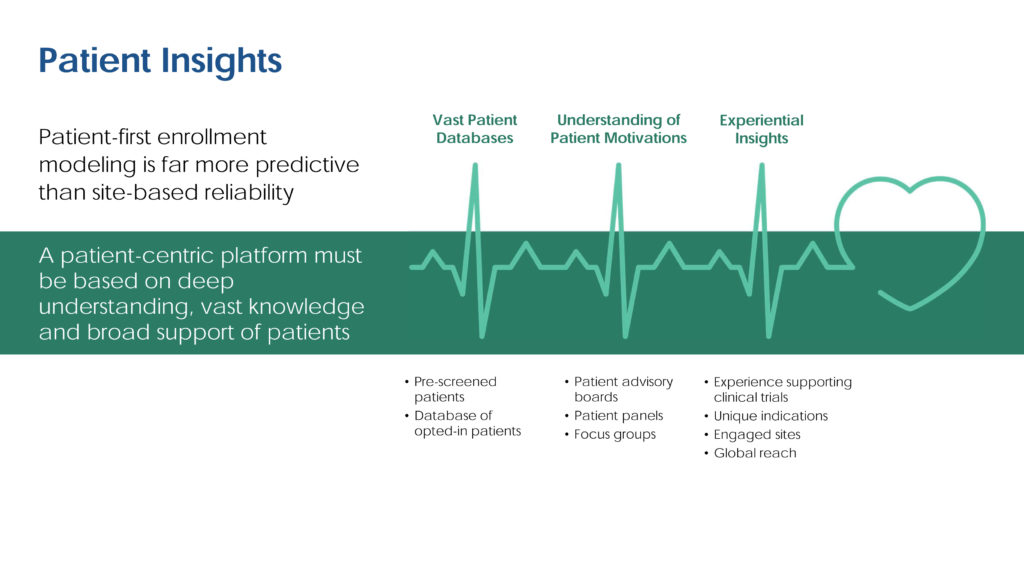 Immensely beneficial to the pharmaceutical industry, this kind of big data can be mined very cost efficiently and leveraged across myriad therapeutic areas and indications that require large volumes of trial candidates. While not easy to achieve, structured, accessible, and very patient-focused data support valuable modeling capabilities. Based on historical experience, protocol specifications, and the indication profile, top-tier PROs are able to target individuals with pinpoint accuracy.
Immensely beneficial to the pharmaceutical industry, this kind of big data can be mined very cost efficiently and leveraged across myriad therapeutic areas and indications that require large volumes of trial candidates. While not easy to achieve, structured, accessible, and very patient-focused data support valuable modeling capabilities. Based on historical experience, protocol specifications, and the indication profile, top-tier PROs are able to target individuals with pinpoint accuracy.
Beyond the demographics of those who have been diagnosed with an indication, this type of “insights to outcomes” patient profiling allows sponsors to predict which individuals are most likely to qualify and participate in a trial. This is an important distinction, as the profile of those who participate in a trial often differs significantly from that of those who simply have the condition.
2. Develop targeted messaging using the patient profile.
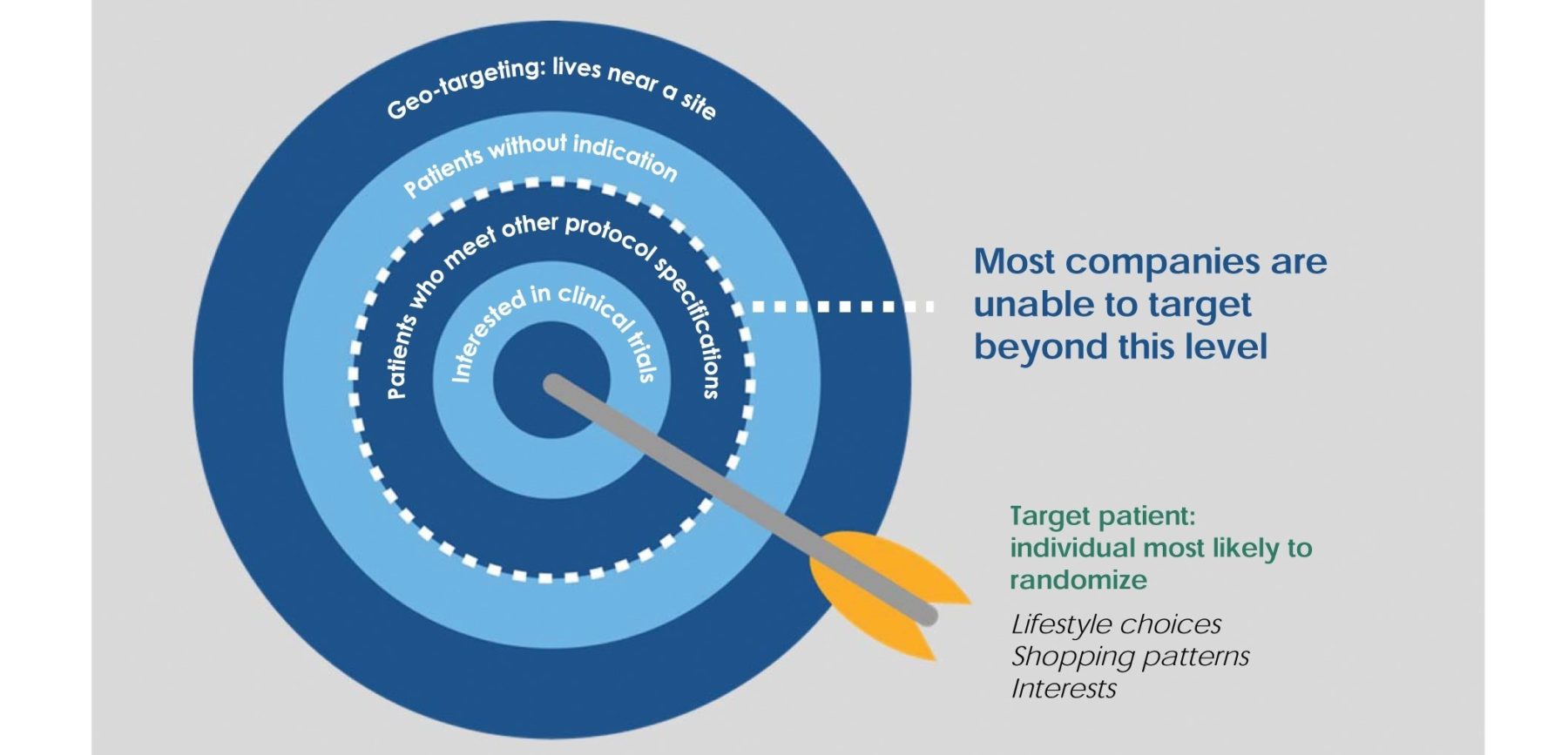
Once the patient profile has been identified, creating awareness for a specific clinical trial can best be achieved through compiling, reviewing, and analyzing patient demographics, financial information, lifestyle choices, interests, shopping and purchasing patterns, and more (see Figure 2). The clinical and lifestyle data can then be combined with profiles of people who previously entered similar studies. This leads the PRO to model the ideal study candidate. The models can be continuously updated as project data is collected, and then applied across various marketing tactics or external databases.
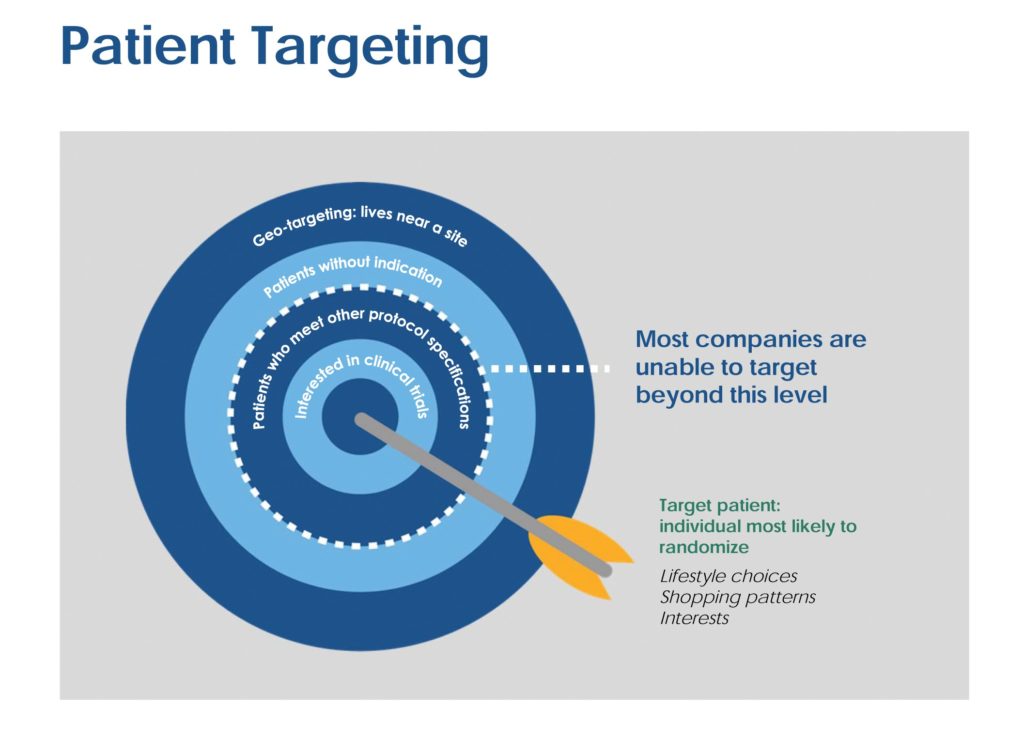 To create strong “lookalike” models that fit the parameters of randomized patients, it is important to determine how the different characteristics interact with each other. This analysis occurs before any outreach is conducted and is specifically tailored to the marketing plan. While most recruitment campaigns randomly blanket the media with general ads in the hopes that qualified patients will respond, it is far more effective to upload the target list into social media platforms and directly communicate with patients via tailored recruitment messages. This strategy is designed to exponentially expand audience reach beyond the database to patients with similar profiles.
To create strong “lookalike” models that fit the parameters of randomized patients, it is important to determine how the different characteristics interact with each other. This analysis occurs before any outreach is conducted and is specifically tailored to the marketing plan. While most recruitment campaigns randomly blanket the media with general ads in the hopes that qualified patients will respond, it is far more effective to upload the target list into social media platforms and directly communicate with patients via tailored recruitment messages. This strategy is designed to exponentially expand audience reach beyond the database to patients with similar profiles.
Patient outreach also can be customized for each protocol based on feedback from indication-specific online groups. By working with these support groups, influential blogs, and global advocacy groups, PROs can reach the appropriate communities with information about the trials and measure their feedback to maximize the impact of the targeted creative concepts.
Advocacy outreach is considered only one part of a comprehensive multi-tactic recruitment campaign. However, it’s still an important way to tap into well-connected communities. As trusted resources, advocacy and support groups offer unique, targeted opportunities to reach potential patients in order to fill studies as they launch.
3. Apply disciplined, constant, real-time monitoring and assessment, and execute swiftly on the incoming data.
Continual testing of marketing concepts, from prior to project launch through the life of the campaign, helps to ensure optimization of recruitment efforts. For example, as noted above, a PRO can test and optimize creative content of recruitment campaigns to determine which graphics, colors, fonts, buttons, and call-to-action messages are most appealing to respondents and are successful at pulling in referrals. Multivariate testing, which focuses on different combinations of elements, is used to identify not only what people say they like, but what they actually do. In terms of response behavior, this is ideal for establishing effective campaign target groups.
However, clicks on a website or online ad don’t automatically translate to randomized patients. It is essential to track all the way through the recruitment funnel, in real time, and focus on the endgame. Remember, a patient views the entire journey from first engagement to randomization as a holistic process.
Essentially, the PRO must act as gatekeeper, controlling the perimeters of the recruitment funnel. The goal is to filter out the majority of respondents who do not qualify, while capturing the remaining patients who self-report on the necessary criteria for:
- Therapeutic targeting: Meeting the appropriate age range and health conditions.
- Geographic targeting: Located within a reasonable distance of research sites.
- Patient motivation: Seeking health and clinical research information.
- Valid contact information: Providing a current phone number and address.
A PRO should be watching everything, every day. This is no small task and requires a unique combination of patience and real-time decision-making to see the best results. This type of strategy relies on measurement and data collection over a period of time, adjusting as data becomes more mature over the duration of the recruitment period.
Know How to Measure Success
A PRO should begin with the end in mind. That means establishing and monitoring metrics at the outset of a recruitment campaign. Metrics can include anything that affects the patient experience, including pre-screener performance, site performance, consents and randomizations, projected timelines, costs, and more. For example, if the sites are not properly prepared to process referrals, the most compelling campaign in the world will not deliver the desired result of randomized patients.
 Patient-centric feasibility can accurately predict how and when patient randomizations happen by combining:
Patient-centric feasibility can accurately predict how and when patient randomizations happen by combining:
- Tens of millions of proprietary patient data points.
- Quantitative/qualitative input from actual patients via large surveys, one-on-one interviews, and/or a patient advisory board.
- Third-party disease metrics.
- Historical site performance metrics.
- The sponsor’s protocol design.
Savvy PROs should employ study-specific enrollment algorithms instead of relying on what sites think they can deliver. Then the PRO and sponsor must monitor every dollar spent to see if they are cutting the time/cost curve by keeping patients at the center of the trial, thus reducing the sites, countries, and study anomalies.
Conclusion
There are many variables that can impact the success or failure of a recruitment campaign—from marketing efforts and site performance, to macro events that are not directly related to the trial. Having clear goals and milestones, persistently monitoring performance, and making changes swiftly to optimize results are the keys to better trial awareness and recruitment. Because clinical trials can lead to lives changed and people saved, it’s worth striving for improvement wherever possible.