Med Device: Remote-Controlled Drugs?
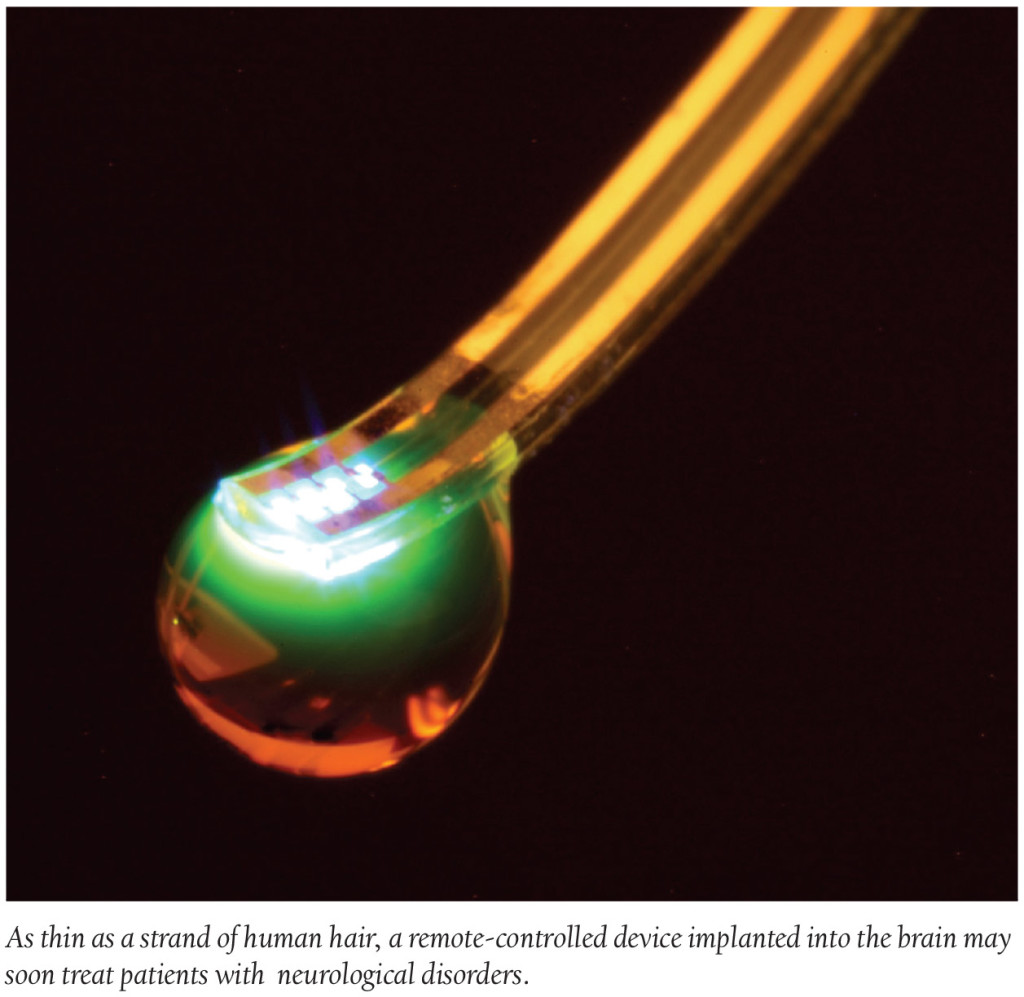
In the future, taking your daily dose of medication may not require a glass of water to wash it down. Instead, medication dosage could very well be released appropriately by remote control. In fact, researchers at Washington University School of Medicine in St. Louis actually engineered a remote-controlled wireless device—only about the width of a strand of hair—that can be implanted in the brain to deliver drugs. The implant is soft, like brain tissue, and is comprised of four chambers that can administer drugs with the push of a button.
The intended use for the device is to help treat patients who suffer from neurological disorders such as depression, epilepsy and pain. The implant works by delivering meds—using light—to targeted parts of the brain. Michael R. Bruchas, PhD, Associate Professor of Anesthesiology and Neurobiology at Washington University says, “With one of these tiny devices implanted, we can theoretically deliver a drug to a specific brain region and activate that drug with light as needed.” This type of delivery method could potentially deliver a more targeted approach for neurological treatments and, researchers note, decrease the severity of medication side effects.
Therapeutic Talk: Ultrasound May Heal Skin Wounds
With a little help from ultrasound technology, hard to heal bedsores and skin ulcers may become a thing of the past for the elderly and those with diabetes. Researchers at the U.K.’s University of Sheffield’s Department of Biomedical Science and Bristol discovered a wound healing method that uses low-intensity ultrasound waves. The ultrasound waves transmit a vibration through the skin, according to researchers, that stimulates the cells in wounds and ultimately accelerates the healing process.
The lead author of the study, Dr. Mark Bass of Sheffield’s Centre for Membrane Interactions and Dynamics (CMIAD), writes, “Ultrasound wakes up the cells and stimulates a normal healing process.” Since the use of ultrasound technology for wound healing is a relatively risk free treatment method, researchers expect it to be clinically tested within the next three to four years.
Doctor Docs: BCBSIL Practice Advance in Development
Blue Cross Blue Shield of Illinois partnered with DuPage Medical Group (DMG), the largest independent physician practice in the Chicago area (with 425 doctors), to develop an innovative value-based care model that benefits both physicians and patients. The new model, which was named BCBSIL Practice Advance, is a value-based program that aids independent physicians in providing their patients with quality, cost-effective care. Designed to pay doctors based on their quality of care and performance measures with their patients, the program is said to ensure practices that benefit both parties. Essentially, the better the quality of services and preventative measures provided by independent doctors, the more the physicians will be reimbursed. The idea is to provide better managed-care for patients, which will ultimately prevent costly hospitalizations for patients.
Drug Development: 3D Printed Epilepsy Drug Allows Higher Dosages
Using 3D printer technology, Aprecia Pharmaceuticals developed Spritam (levetiracetam), a drug for seizure prevention in epilepsy patients. The new technology allows Aprecia to manufacture higher doses of the medication—up to 1,000 milligrams.
How? The 3D printing machine builds the pill by producing one layer of medication, and topping that layer with others continuously until the programmed dosage is reached. This makes the oral medication porous and customizable, easy for epilepsy patients to swallow, and can also be individually packaged so consumers don’t need to worry about measuring dosage. According to CNN, the FDA recently approved the new drug—the first 3D drug ever approved—and it will be available for adults and children within the next year.
Discoveries/Innovations: “Play Dough” Accelerates Fracture Healing
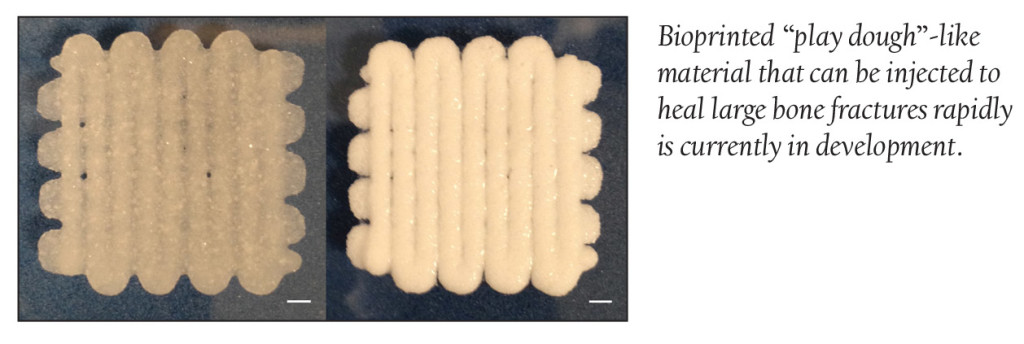
Scientists at the University of Nottingham recently developed a new technique capable of healing large fractures at an accelerated rate. The technique involves ambient temperature bioprinting of a strong “play dough”-like material. The bioprinting material is composed of a blend of poly (L-lactic-co-glycolic acid) and polyethylene glycol that form a micro-particulate paste that is extrudable and moldable and capable of incorporating protein-releasing microspheres.
Scientists demonstrated how the micro-particle paste can be injected by syringe and can sustain stresses and strains similar to cancellous bone, the spongy bone tissue found at the end of long bones. This method of bioprinting is said to be a viable route to the production of materials that make for an accelerated bone repair.
FDA Update
Drug Approvals
The FDA recently approved Genentech’s CellCept, a drug for those with recent kidney heart or liver transplants. The drug, taken with other anti-rejection medications, weakens the patient’s immune system to keep it from rejecting the foreign, implanted organ.
The FDA has approved Bristol-Myers Squibb’s Droxia. The oral medication may help those with various types of cancer including leukemia, skin cancer or ovarian cancer.
Galderma’s Epiduo Forte, a topical prescription drug for acne treatment received FDA approval. Galderma claims more than half of their study pool saw marked improvement in facial acne after one week of use.
Sprout Pharmaceuticals’ Addyi is the first treatment approved to boost a woman’s libido. More specifically it is a treatment for generalized hypoactive sexual desire disorder (HSDD), the most common form of female sexual dysfunction, affecting up to 1 in 10 women.
Amneal Pharma’s drug, quinine sulfate, recently received FDA approval. The oral medication will now be available to treat malaria. It is approved to treat uncomplicated forms of the disease in adults and is said to be effective in regions at which an immunity has been built up to other malaria therapies.
Medical Device Approvals
The first Osseoanchored Prostheses for the Rehabilitation of Amputees (OPRA), manufactured by Sweden-based Integrum AB, received approval for usage in patients with above the knee amputations. The device is designed for those whose limb is not long enough for socket prostheses. The OPRA device requires two surgeries for implantation; the first to attach the base to the thigh bone and the second six months later when enough tissue has grown to anchor the rest of the leg prosthesis.