Therapeutic Talk: Medical Breakthrough Helps Paralyzed Patients Walk

The latest and most dramatic advancement in spinal cord stimulation has already helped five patients who were completely paralyzed from the waist down to walk again. This breakthrough has proven that an injury interrupting brain signals to the spinal cord can be overcome by retraining the spinal cord independently. “It might be that the spinal cord can act on its own, almost completely without signals from the brain,” Susan Harkema of Kentucky Spinal Cord Injury Research Center and leader of the development team on this project told NBC News in an interview. “It can relearn.”
Epidural stimulation requires a device to be implanted in the epidural at the location of disconnect from the brain, where 16 electrodes are then connected to the nerves that control sensorimotor signals to the legs. A battery unit is implanted inside the abdomen to power the stimulator and allow it to be controlled wirelessly. The patients then go through a very specialized physical therapy routine where professionals manually move the legs to mimic the motions of walking. When the spinal cord has relearned enough, the patient can move to the treadmill and slowly practice walking each day.
Of the five patients currently undergoing epidural stimulation, three can walk with walkers alone while two still need assistance from therapists. The stimulator does not fix the problem of paralysis, but rather tries to bypass the brain’s disconnect, meaning the treatment doesn’t work for everyone all the time. Nonetheless, patients who have been paralyzed from the chest or waist down for years are now able to get out of bed and walk across a room again.
Medical Device: Post-Surgical Pumps Get a Much-Needed Update

SOMAVAC Medical Solutions has revolutionized the inefficient and inconvenient post-surgical pump patients have been forced to use since the 1970s. The SOMAVAC 100 applies a sustained vacuum to a closed wound following surgery to remove fluid effectively and reduce the risk of seroma. The low-profile, user-friendly device can be discreetly worn under clothing which helps patients return to normal activities while recovering. It is currently the only alternative to the suction bulbs used in closed suction drains.
Surgical drains are worn by patients anywhere from days to several months depending on the self-reported fluid volume. SOMAVAC quotes a breast cancer survivor commenting on manual pumps as saying, “Doctors should consider quality of life with drains as part of their decision-making criteria. If they were walking around with uncomfortable hoses and bulbs hanging out of their armpits with blisters and chafing all over, I’m sure they’d rethink their opinions about them in a hurry.”
Without providing continuous suction, the manual pumps often prolonged drain indwelling times and increased opportunities for complications. Bulbs are also fraught with issues such as leakage, clogging, and spillage, and patients struggle to follow instructions which may lead to inaccurate use of drains. The FDA approved an automated, wearable, post-surgical pump for use in a range of surgeries which lead to large surgical flaps requiring drains, such as abdominal surgery, mastectomy, cosmetic surgery, hernia surgery, orthopedic procedures, etc.
TeleMed Texts: Apple Watch Transforms into a True Medical Wearable
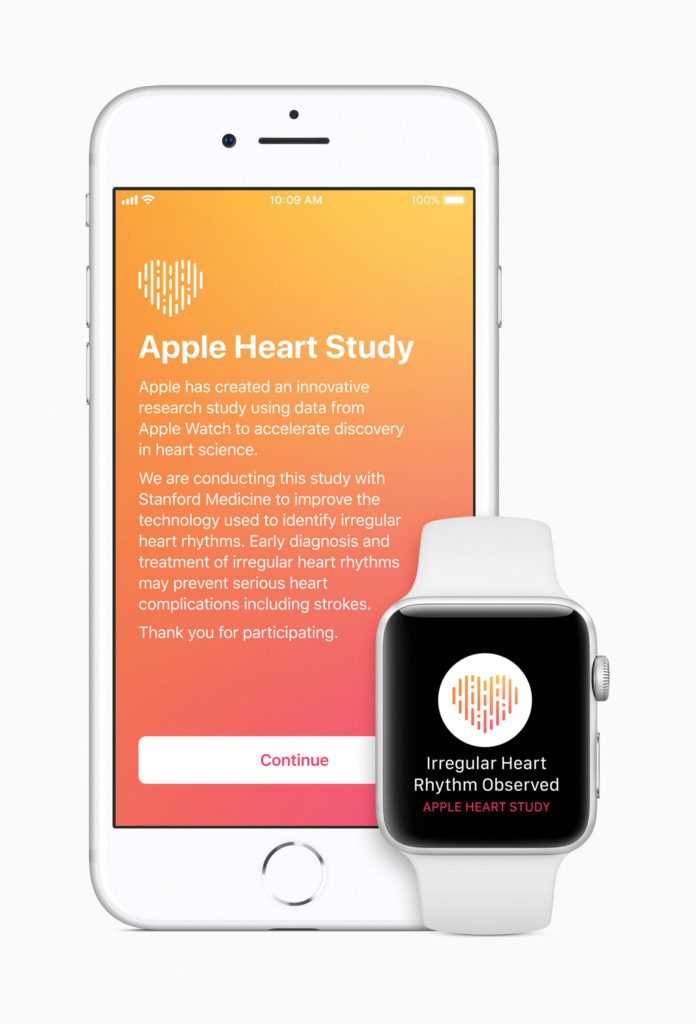
Moving away from popular fitness tracking features, the Apple Watch received FDA approval for an app dedicated to using the heart rate tracker to detect atrial fibrillation, an irregular heart rhythm condition that can lead to blood clots, stroke, and heart failure. The Apple Watch Series 4 will now come standard with an electrocardiogram (ECG) monitor in addition to the typical IR sensor. Electrodes will conduct an ECG test when you open the app and touch your finger to the watch.
Apple has met a serious need for the 2.7 million Americans with A-fib, as it can be extremely difficult to diagnose people with irregular heart rhythms. Current devices are often bulky or invasive, making people less likely to want to wear them while only monitoring heart rate for a temporary period of time. “Having a device that the patients already use as part of their everyday life and have with them at all times, where they can very quickly have a symptom and very quickly generate an EKG can be extremely helpful,” Dr. Adriana Quinones-Garcia, a cardiologist at New York University’s Langone Health, tells Popular Science. The fact that Apple is willing to work with the FDA and fill niche gaps in healthcare tech markets shows their eagerness to disrupt the medical device industry just as it has done with phone, music, and other tech industries.
Trend Setting: A New Way to Manage HIV Emerges
While HIV patients can now manage their symptoms and live a healthy life, antiretroviral therapy requires patients to take oral medications every day with the ever-present risk of dangerous viral rebounds should they deviate from their regimin. However, new research has revealed a method of controlling HIV virus levels for months at a time, changing the rigorous adherence routine HIV patients often struggle with. New clinical trials from Rockefeller University researchers suggest that a combination of two anti-HIV antibodies, called broadly neutralizing antibodies or bNAbs, are both safe and more effective than any previously tested antibody immunotherapy.
“The expectation is that these new variants will have three- to four-fold longer half-lives,” said Rockefeller’s Michel Nussenzweig, a co-leader in both bNAb studies, in a statement. “So we may be able to give the antibodies once or twice a year.”
In addition to HIV treatment, bNAb therapy could revolutionize HIV prevention. Currently, people at risk for contracting HIV can take preemptive antiretroviral medication, which also requires daily dosages. But bNAb therapy has the added benefit of remaining in the body longer than antiretroviral drugs, and therefore should require less frequent administration. Ultimately, long-term HIV medication would allow people to better prevent and avoid spreading HIV without adhering perfectly to pill taking.
Discoveries/Innovations: Oncoceutics Takes Novel Approach to Cancer Therapies
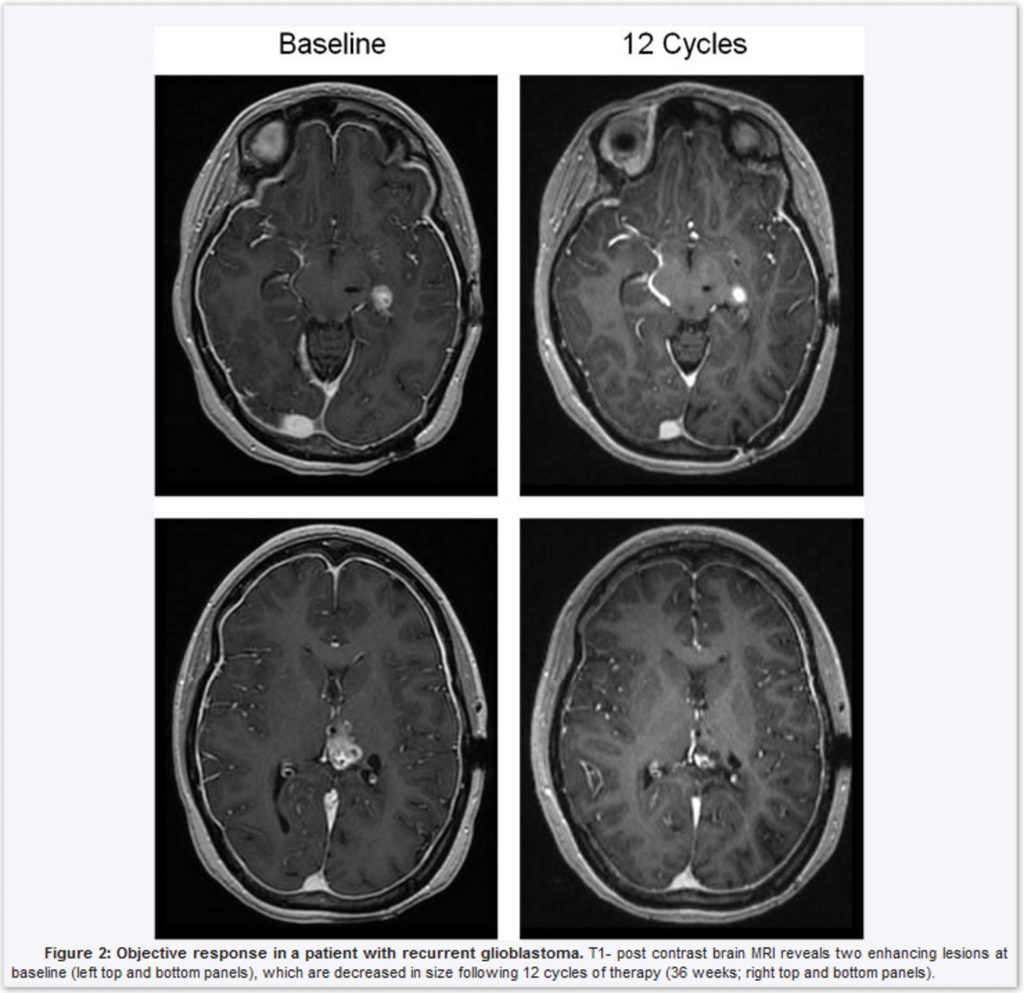
The cancer-focused drug and development company has built a portfolio of unique cancer therapies that target G-Protein Coupled Receptors (GPCR), a class of molecules contributing to cancer development that was previously deemed “undruggable.” The dopamine receptor DRD2 has been recognized as a main component of glioblastoma, a lethal cancer, along with the anti-cancer effects of targeting DRD2 with Oncoceutic’s lead compound ONC201. “It is exciting to see the utility of additional imipridones beyond ONC201 for the treatment of lethal cancers with no curative treatments, such as glioblastoma,” said Markus Siegelin, MD, Assistant Professor of Pathology & Cell Biology at Columbia University who served as a senior corresponding author of the publication. “Our study provides unique insights into the mechanism of DRD2 antagonism resulting in the identification of biomarkers for the imipridone clinical program.”
The oncology therapy developer has published a scientific research article in Clinical Cancer Research outlining these uses for an imipridone portfolio of compounds in neuro-oncology as well as the introduction of ONC206 into the clinic in 2019 to treat gliomas because of the connection between DRD2 antagonism to downstream signaling effects that cause cancers. “The imipridone family of compounds contains several new therapeutic concepts for oncology that build out from our understanding of ONC201,” said Joshua Allen, PhD, Senior Vice of President R&D at Oncoceutics, in a statement. “These exciting new findings set the stage for the upcoming clinical introduction of ONC206 as the next imipridone to make clinical impact.”
FDA Update
Drug Approvals
Pfizer receives FDA approval for their new dacomitinib tablets Vizimpro, a first-line treatment for patients with metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 L858R substitution mutations as detected by an FDA-approved test.
Breakthrough Orphan Drug Designation
The FDA approves breakthrough and orphan drug designation for Poteligeo, manufactured by Kyowa Kirin. The intravenous injection is to be used for the treatment of adult patients with relapsed or refractory mycosis fungoides (MF) or Sézary syndrome (SS). MF and SS are types of non-Hodgkin lymphoma in which lymphocytes become cancerous and affect the skin, causing itchy red rashes and skin lesions and can spread to other parts of the body. Poteligeo is the first drug to receive FDA approval specifically for SS, a rare form of skin lymphoma that affects the blood and lymph nodes.
Competitive Drug Designation
The FDA grants Competitive Generic Therapy (CGT) designation to potassium chloride oral solution USP to Apotex Inc. USP is used for treatment and prevention of hypokalemia (low potassium blood levels) in patients who are on diuretics, and those for whom dietary management with potassium-rich foods is insufficient or diuretic dose reduction is not possible.
Medical Devices Approvals
ImpediMed has received FDA clearance for SOZO, the world’s first bioimpedance spectroscopy (BIS) device to monitor a heart failure patient’s fluid status in a clinical or at-home setting. SOZO is the most detailed and accurate BIS device available, designed to create a customized plan around individual patients.






