Med Device: Walking Made Easy
The act of walking may seem simple enough, but people expend more energy during walking than any other daily activity. Every bit of that energy matters to the elderly and those with mobility issues. But now walking can be much more efficient.
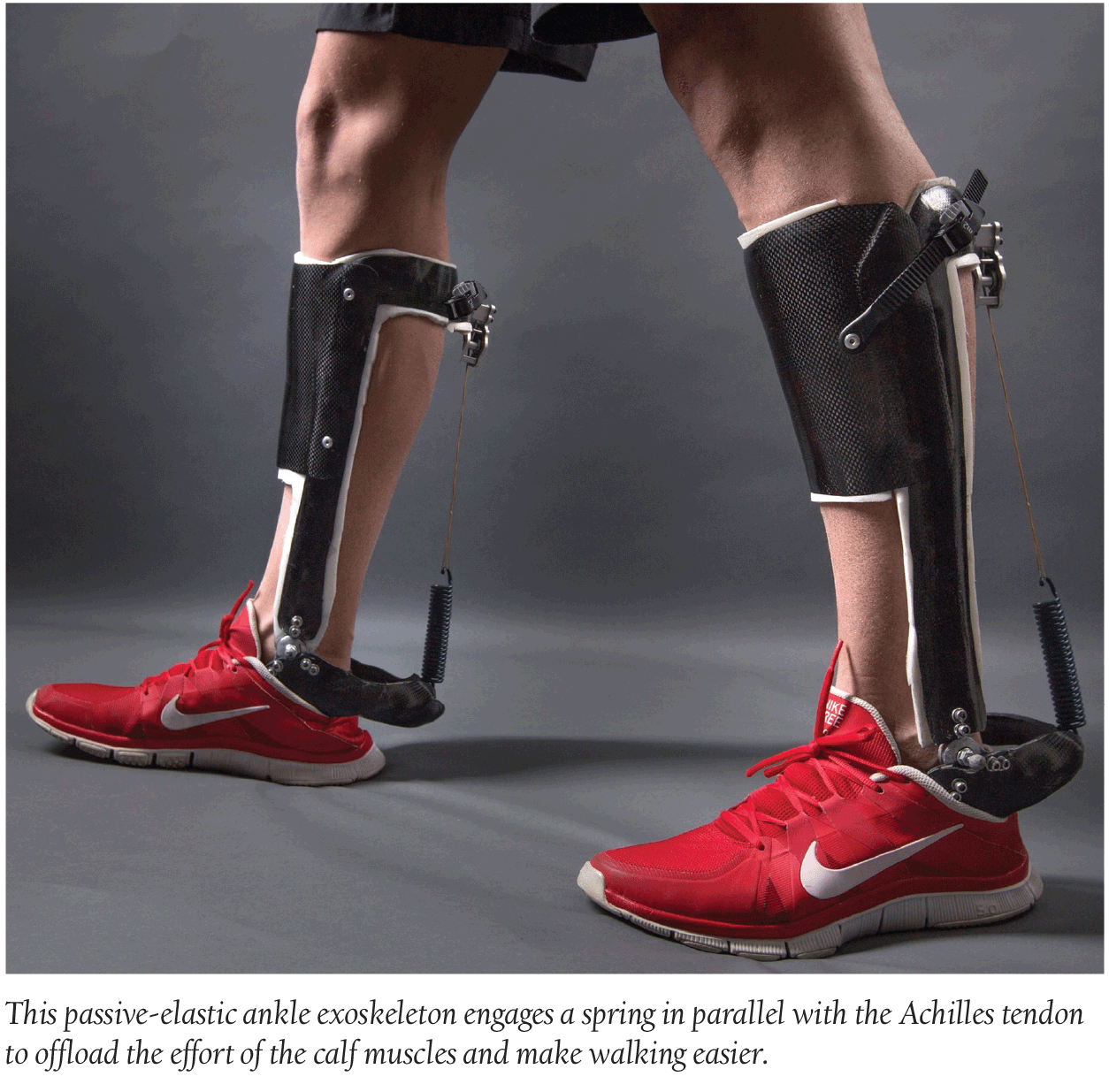
Researchers from Carnegie Mellon University and North Carolina State University recently revealed an unpowered ankle exoskeleton that reduces the metabolic cost of walking by approximately 7%—or roughly the equivalent of removing a 10-pound backpack. The device works by taking over the effort of the calf to produce force without consuming energy.
“You can imagine these lightweight efficient devices being worn on the affected limb to help people with the permanent after effects of stroke,” Steve Collins, an Assistant Professor of Mechanical Engineering at Carnegie Mellon, said in a statement. “We’re hopeful that designs that use similar techniques can help people who have had a stroke walk more easily. We’re still a little ways away from doing that, but we certainly plan to try.”
Discoveries/Innovations: Nicox Presents Promising Glaucoma Formulas
An international ophthalmic company, Nicox, developed two new nitric oxide donors for treatment of glaucoma or ocular hypertension. NCX 667 optimizes nitric oxide dosing—proving to reduce intra-ocular pressure in preclinical trials, which made it the most innovative research project at the Association for Research in Vision and Ophthalmology (ARVO) 2015 Annual Meeting. NCX 470 is a new, improved nitric oxide-donating bimatoprost, a leading intra-ocular pressure reducer.
“Our scientists are now working on other potential next-generation compounds for glaucoma,” says Dr. Michael Bergamini, Chief Scientific Officer and Executive Vice President at Nicox, “with two sets of encouraging results announced last week at the ARVO Annual Meeting, the world-leading research conference in ophthalmology.”
Therapeutic Talk: Bayer Calls for Multi-National Antibiotic Research
The German pharma company, Bayer, hopes the seven wealthiest nations will collect $22.4 billion dollars to produce antibiotics to combat the impending threat of super-resistant bacteria.
“I expect a multinational fund for antibiotics research,” CEO Marijn Dekkers stated in Germany’s Der Spiegel magazine. “One country alone can’t shoulder it.”
The World Health Organization declared the rise of resistant “superbugs” as a serious challenge in the infectious disease field of research—Bayer expects the Group of Seven (G7) nations to fund the search for a solution. Private sectors failed to fund this type of production because the pharma industry claims these drugs would only be used in very few instances in which all other therapies fail to avoid furthering the resistance of future generations of bacteria.
Trend Setting: MinuteClinic Reaches New Milestone
Beginning as a single walk-in medical clinic in 2000, MinuteClinic offered fast, convenient healthcare and it’s now a service in high demand. MinuteClinic, acquired by CVS in 2006, surpassed 25 million patient visits since its opening.
CVS Health plans to help that number grow even higher, opening 100 new clinics this year. The company anticipates surpassing 1,500 total clinics by 2017. The clinic also hopes to further serve patients through the integration of electronic health records to better communicate with patient’s physicians, CVS pharmacists and the 50-plus hospitals they collaborate with across the U.S.
“The need for more accessible and affordable retail clinic healthcare services is greater than ever as the medical profession addresses the primary care shortage, changes brought by healthcare reform and the needs of our aging population,” Andrew Sussman, MD, MinuteClinic President and Executive Vice President/Associate Chief Medical Officer, CVS Health, said in statement. “As we look to the future, we will continue to expand our service offerings.”
Study Sessions: Eskenazi Health Collaborates to Personalize Medicine
Eskenazi Health is working with the Indiana Institute for Personalized Medicine, the Indiana University School of Medicine and the Regenstrief Institute to study pharmacogenetic results, or the role that genes play in a patient’s response to drug therapy. The companies hope to find ways for physicians to personalize medications and improve results based on the patient’s genetics and personal responses to treatment.
Since drugs are developed for the average individual, they don’t always work best for a portion of patients. This study hopes to also reveal if personalization will reduce hospital and outpatient costs, adding an economic benefit to better patient care. A total of 6,000 patients will be investigated in all medical aspects for one year, testing 33 different drugs and their medical costs.
Dr. David Flockhart, Director of the Indiana Institute for Personalized Medicine and principal investigator, said in a statement, “Scientists will test the utility of this approach in a community-based setting, and in rural, underserved and economically disadvantaged populations. We need to figure out not only whether using genomics in the clinic can be helpful to patients, but also if it will be cost effective.”
According to David S. Karow, MD, PhD, assistant professor of radiology at UC San Diego and the study’s author, the technique will allow doctors to more precisely and effectively determine treatment.
FDA Update
Drug Warnings
The FDA has issued a warning against SGLT2, a class of diabetes drug that has likely led to ketoacidosis cases in users, sending them to the emergency room. SGLT2 inhibitors include Johnson & Johnson’s Invokana and AstraZeneca’s Farxiga.
Drug Approvals
FDA approved Bayer’s Avelox to treat two forms of plague—a rare, sometimes fatal bacterial infection found last year in Colorado and possibly spread through human-to-human contact.
Merck KGaA received Fast Track designation for the development of evofosfamide, currently in Phase III trials. Under development in collaboration with Threshold Pharmaceuticals, Inc., the drug treats previously untreated patients with metastatic or locally advanced unresectable pancreatic cancer.
Med Device Approvals
The FDA approved Greatbatch Medical’s MyoPore sutureless myocardial pacing lead to help the heart beat at an appropriate rate. The device is implanted at the base of the heart and acts as an electric pacemaker for those with heart failure symptoms who cannot support other kinds of cardiac therapy implants.
Medtronic’s Model 5071 lead received approval for use in conjunction with cardiology devices such as pacemakers, defibrillators and CRTDs, which combine the benefits of pacemakers and defibrillation therapy.
Panel Approval
The FDA advisory committee voted 12-1 to recommend the approval of Vertex Pharmaceuticals’ experimental cystic fibrosis drug Orkambi. Vertex’s study shows that Orkambi, intended for patients 12 and older, is more effective in improving lung function due to its addition of lumacaftor. The FDA will make their final decision regarding approval by July.