VYVGART®
argenx
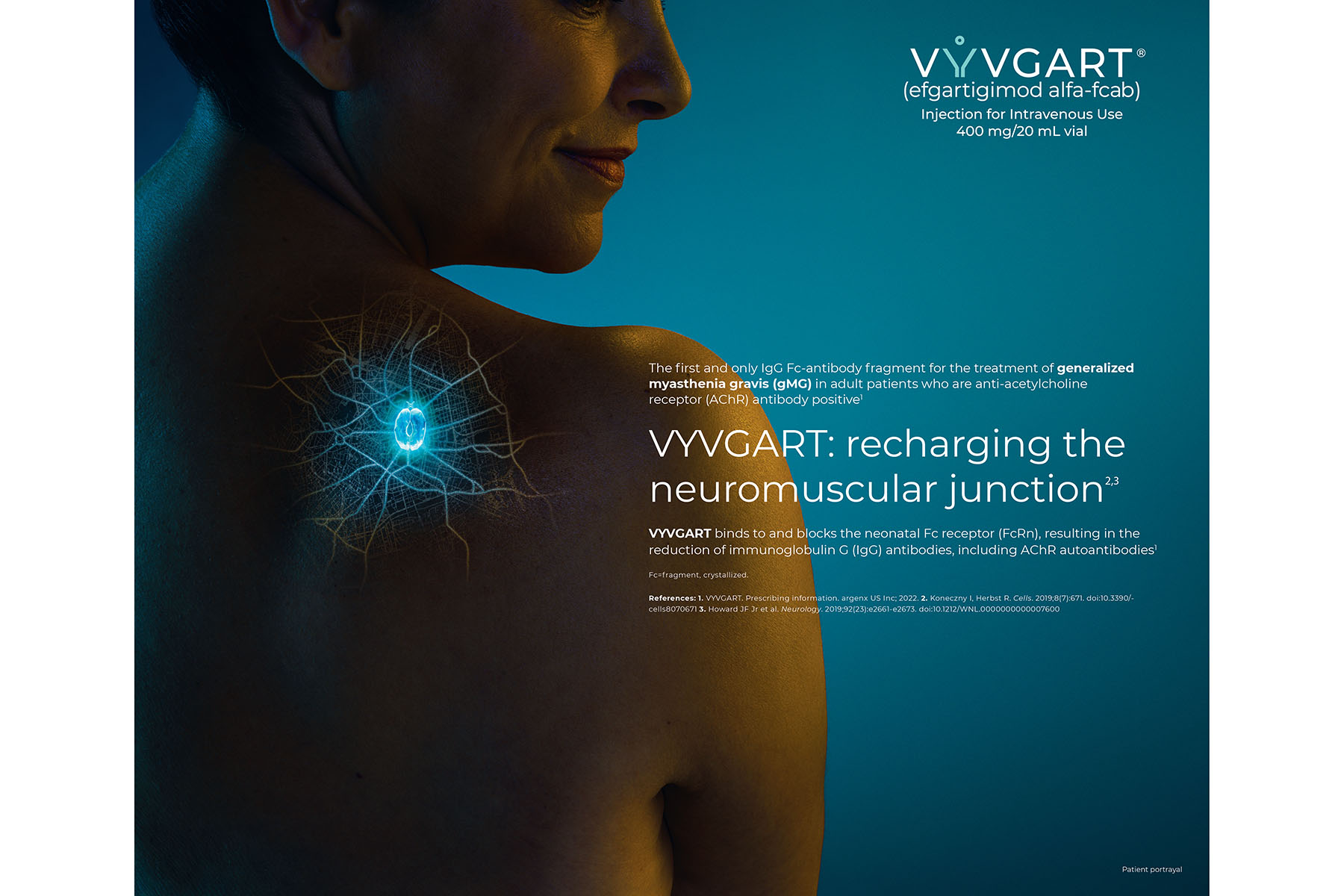
VYVGART (efgartigimod) is a pioneering, first-of-its-class medicine that is helping to fill an urgent unmet clinical need for adults with generalized myasthenia gravis (gMG), a rare, chronic neuromuscular autoimmune disease. argenx’s development of VYVGART was based on years of close collaboration with patients and the groundbreaking scientist, Prof. E. Sally Ward, PhD, who discovered the medicine’s underlying mechanism. The product is a triumph of innovative immunology and antibody engineering, and gMG is just the start; efgartigimod has the potential to treat more than a hundred other autoimmune diseases mediated by the same antibody as gMG.
VYVGART is the first medicine that addresses a root mechanism of IgG-mediated neuromuscular degradation, rather than broad immunosuppression commonly seen with existing autoimmune disease treatments and as such has been rapidly adopted by patients in its first months of commercial availability. It is the first and only neonatal receptor (FcRn) blocker approved by the FDA, establishing and leading a new class of drugs. Prior to the approval of VYVGART, there were limited safe and effective FDA-approved medicines for this disease. VYVGART is a major innovation in gMG treatment, representing new hope for tens of thousands of patients in the U.S. alone.
gMG imposes a significant burden on patients and their loved ones. People living with gMG may experience difficulties with facial expression, speech, swallowing, vision, and mobility—for some, this means daily activities such as laughing, smiling, or even getting out of bed can be a significant challenge. In life-threatening cases, gMG affects muscles responsible for breathing, which can lead to hospitalization. Symptoms vary from patient to patient, and in the same patient at different times. This unpredictability takes a serious toll on patients’ quality of life and psychological well-being. Many patients struggling with this disease are also diagnosed with depression and/or anxiety.
Roughly 17,000 patients with gMG in the U.S. have been inadequately managed with commonly used therapies. Many patients have experienced suboptimal treatment outcomes, which has led to a forced complacency in both HCPs and patients. However, VYVGART is offering new hope to patients like these. The product has only been available for less than half a year, but there is already promising adoption among eligible gMG patients that prove it is filling an unmet need in the industry. And there are reports of patients who are achieving activities that previously felt impossible due to this innovative product.