Impel Pharmaceuticals, Inc.
Contact Person:
Jen Berman, Vice President, Marketing
jberman@impelpharma.com
Impel Pharmaceuticals Inc. was founded on the mission to address an unmet need in treating CNS diseases, including migraine. Traditional nasal spray pumps have only targeted the lower nasal space, a route that provides relatively lower absorption. Between 30-40% of migraine patients fail to respond to this treatment—and nearly 80% have reported being unsatisfied with results.1
Impel believed that intranasal drug delivery could improve the uptake and absorption of important molecules. Their proprietary solution, Precision Olfactory Delivery (POD) technology, ensures consistent dosing via a gas-propellant delivery mechanism that eliminates the need for coordinated breathing. The potential for the POD technology is significant as it can deposit both liquid and dry powder formulations. POD technology is scalable so that essentially the same device is used throughout the development program. This opens the door to many potential investigational programs across multiple diseases.
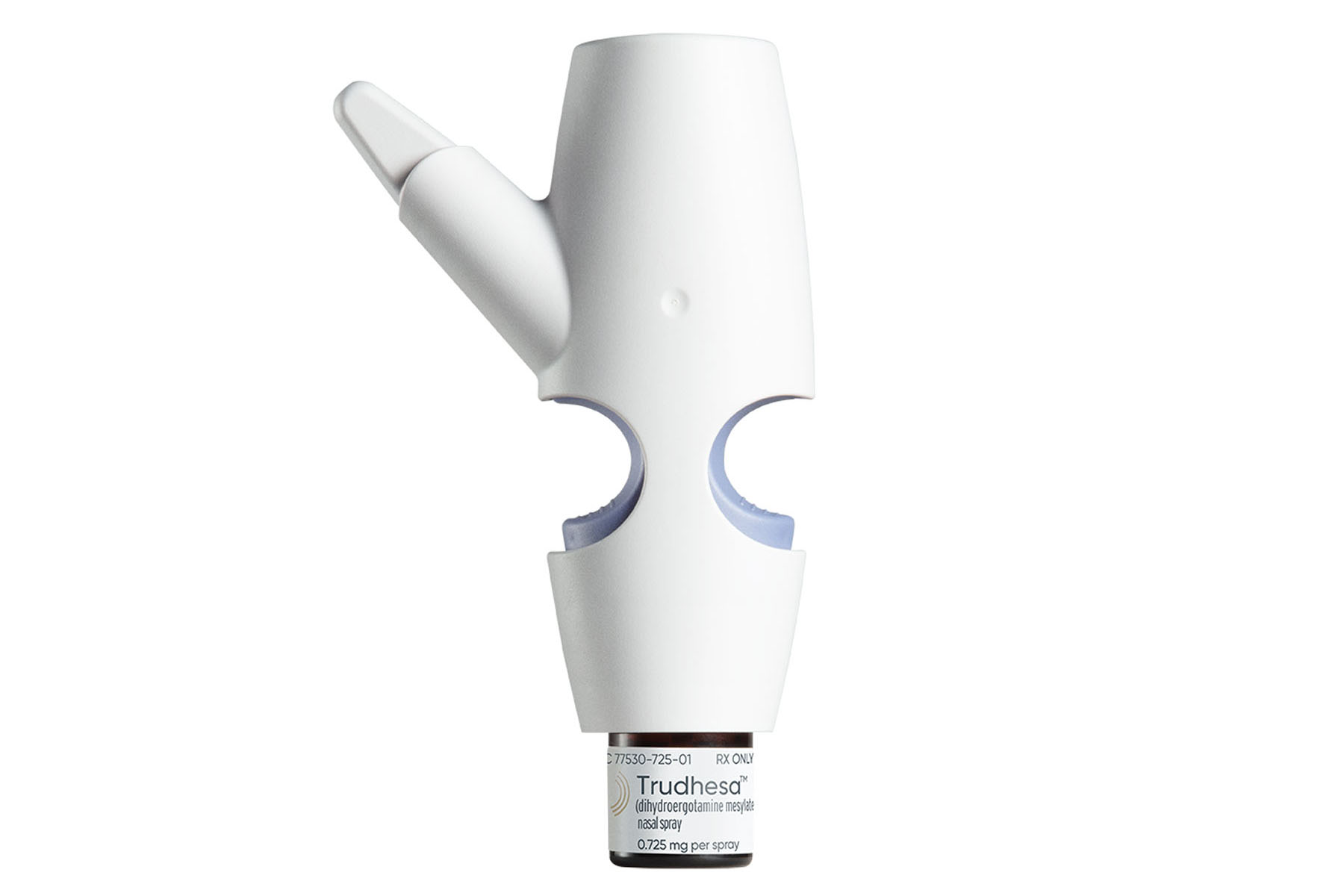 The POD technology has the potential to offer hope for patients living with conditions who have defied effective treatment to date. Its rapid, non-invasive profile may aid the patient-provider experience, particularly in therapy involving those with mental illness.
The POD technology has the potential to offer hope for patients living with conditions who have defied effective treatment to date. Its rapid, non-invasive profile may aid the patient-provider experience, particularly in therapy involving those with mental illness.
For example, in September 2021 Trudhesa (dihydroergotamine mesylate [DHE]) nasal spray) became the first FDA-approved treatment utilizing the POD technology. With the potential to treat patients who have not previously responded to therapy, Trudhesa may lower the need for urgent doctor visits, while providing pain relief without the nausea and vomiting associated with IV DHE treatment for migraine. In a clinical trial that treated 5,650 migraine attacks, 66.3% of patients reported pain relief, some within as little as 15 minutes.
Trudhesa represents a major advance in reducing migraine pain and providing patient satisfaction.
References: