For quite some time healthcare costs have stood firmly in the crosshairs of lawmakers, care advocates, and cash-strapped patients—and not without reason. Obamacare was intended to dampen the growth of healthcare costs, while increasing quality of care and expanding access. However, at 28% of the 2017 U.S. federal budget, healthcare is the largest expense, nearly 40% higher than the defense budget. While industry experts know that drug costs represent just 9-10 cents of every dollar spent on healthcare, prescription drugs remain easy fodder for criticism, especially due to the highly publicized actions of a few “bad apples” and as ever more innovative and complex therapeutics target some of humanity’s most elusive—and costly—diseases.
With each passing tweet, speech, and interview, it has become overwhelmingly apparent that the Trump Administration seeks to make drug cost controls a central tenet of its platform. While it remains unclear what reforms will resemble—the President’s press secretary has assured the public of a renewed promise to negotiate Medicare prices—there is undoubtedly change coming our way. Continued media focus, spurred by Senator Bernie Sanders’ candidacy and successive high-profile price increases, have put biopharma firmly on notice.
Every day, the biopharma and med tech industries work hard to demonstrate the value of their drugs to everybody from payers to patients. Promoting six-figure therapeutics in the face of pricing headwinds is never simple, but savvy marketers demonstrate that it’s possible through creative approaches to everything from packaging to patient advocacy.
To develop the right solutions, marketers must look back to the Triple Aim, a framework first proposed by the Institute for Healthcare Improvement, conceived in the process of drafting the Affordable Care Act of 2010. The central idea is that improvements to care delivery must simultaneously pursue three dimensions: An improvement on patients’ experience of care, advances in population health outcomes, and a reduction in the per-capita cost of healthcare. Regardless of whether or not ACA survives to see 2018, the Triple Aim will live on as a comprehensive scorecard with which to evaluate changes to our healthcare system on all sides: payer, provider, and pharma.
Successful marketers should be mindful of all three dimensions when developing their brands’ commercial strategies. To do so, first consider the three points in the context of pharma:
- Improved Quality of Care. With regard to biopharma/med devices, this could mean greater ease of use, more personalized support, streamlined payment and coupon programs, more simplified reimbursement, or lower rates of product switching, which could be difficult for patients and providers alike.
- Lower Costs. Savings might be accomplished two ways, either through lower drug list prices, or more desirably, greater adherence to necessary medicines that will lower overall cost burden on the healthcare system.
- Better Health Outcomes. Real-world evidence (RWE) that effectively measures relevant health outcomes is becoming a cost of doing business. This includes evaluating costly interactions with the healthcare system (think hospital readmissions), as well as quality of life and productivity metrics.
Digital health offerings, often called “going beyond the pill,” hold the immense potential to further all three dimensions. Abilify’s digital pill is a terrific example. Otsuka is coupling an existing blockbuster drug, Abilify (aripiprazole) for schizophrenia, with a digital sensor embedded inside each pill. Patients, family members, physicians, and social workers will be able to monitor a patient’s habits. Poor adherence is common with antipsychotic therapies, especially among schizophrenic patients. This product, if used correctly, could alleviate issues with adherence, simultaneously cutting costs and preventing dangerous downstream complications.
How Data Helps With Adherence
Otsuka’s digital pill provides physicians with critical information on their patients, and helps them determine or refine their treatment approach. With a few months of adherence data, physicians and social workers will be able to gauge a patient’s progress—and intervene if necessary. There is tremendous value in detecting nonadherence early, not just to patients who are safeguarding their own health, but to the system that would be obligated to pay for their inobservance. Chip-embedded Abilify, which is on track to launch in the next year, will undoubtedly cost more than its generic counterpart. But as physicians and patients will find, there’s immeasurable value in effective treatment and happier patients.
While Otsuka’s digital pill is innovative and intriguing, strategies that seek to fulfill the Triple Aim need not be complex or costly. Often overlooked, innovations in packaging are perfectly capable of improving adherence, especially in drugs taken more than once a day. Thoughtful drug packaging design is a miniscule investment for drugmakers, and one that can encourage patient ownership of their healthcare. The more engaged patients are, the less likely they are to switch, perhaps even in the face of generic options. Compared to post-marketing studies, nationwide coupon programs, and large call centers, innovations in packaging cost next to nothing, but can help to differentiate products from competitors, exude high quality, and ultimately drive higher adherence while lowering overall healthcare costs in the process.
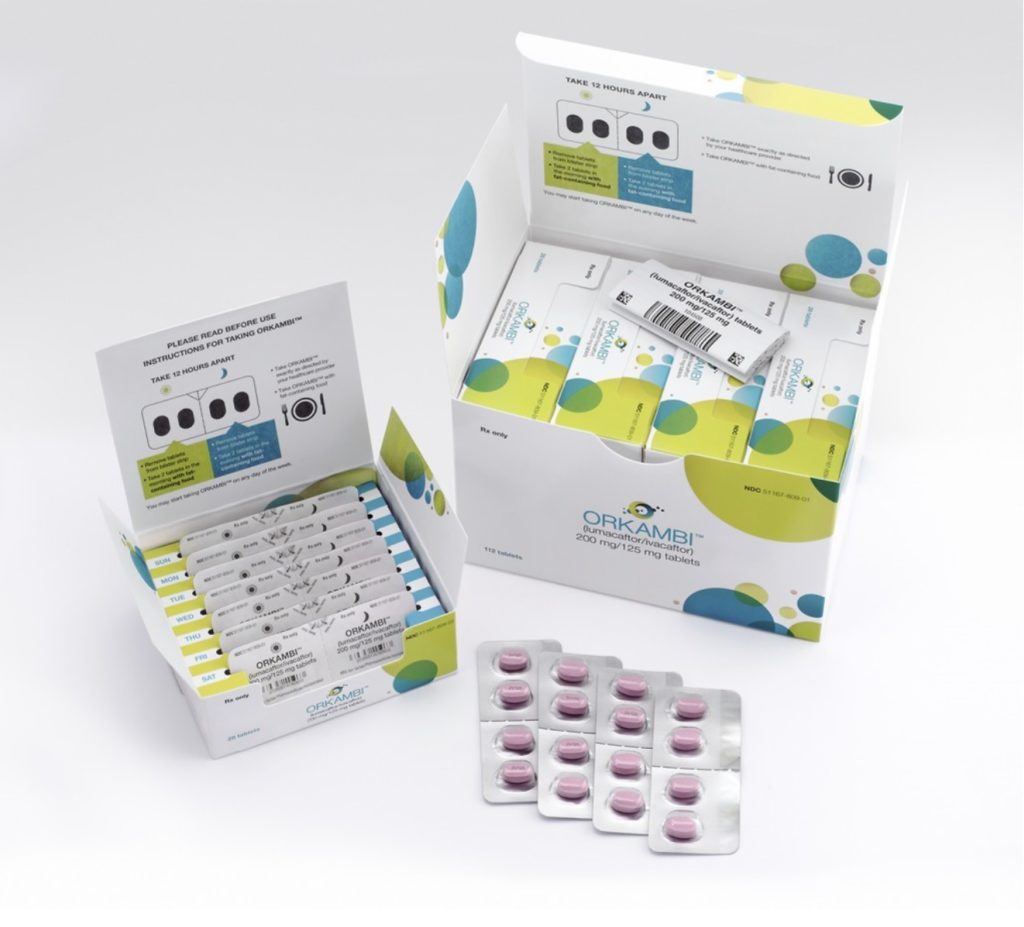
Last year, Vertex Pharmaceuticals was lauded for its packaging of Orkambi (lumacaftor/ivacaftor) for cystic fibrosis, sold in daily blister packs with colorful graphics to highlight separate AM and PM doses. This not only helps to engage patients, it also simplifies an otherwise confusing dosing regimen and allows for further reinforcement of their brand.
 In similar rare-disease spaces, patient affairs is another lever that marketers can pull to improve overall care quality, adherence, and ultimately, cost. Consider the case of Elelyso, an enzyme replacement therapy for Gaucher disease. Pfizer licensed the product from Protalix, an Israeli biotech, with the intention of leveraging their global capabilities in rare disease markets.
In similar rare-disease spaces, patient affairs is another lever that marketers can pull to improve overall care quality, adherence, and ultimately, cost. Consider the case of Elelyso, an enzyme replacement therapy for Gaucher disease. Pfizer licensed the product from Protalix, an Israeli biotech, with the intention of leveraging their global capabilities in rare disease markets.
Realizing that Gaucher patients knew their disease far better than a corporate marketing team ever could, Pfizer recruited real-life Elelyso patients to serve as brand ambassadors. These individuals helped initiate others on the drug, which lowered the physical and emotional costs of drug-switching. Responding to a lack of trust stemming from competitor Sanofi Genzyme’s 2009-2012 shortage of Cerezyme for Gaucher, Pfizer sought to provide a highly personalized treatment experience. Instead of a call center, Pfizer deployed their battalion of ambassadors, commanded by a member of the marketing team in the company’s New York headquarters. Patient visits and regular check-in calls reassured patients, securing both loyalty and adherence in the process.
Because of their strong relationship with Gaucher patients, Pfizer was able to use their deep insights into the patient journey, and identify opportunities to improve the overall care experience. A majority of Gaucher patients are Ashkenazi Jewish, and upon realization that the drug was entirely plant-based, culturally savvy marketers at Pfizer sought a Kosher certification for their product, becoming the first prescription drug ever to receive a Kosher designation from the Orthodox Union. Although the Torah excludes life-saving medicines, Pfizer’s patient affairs liaison acted on the request of their patients. The Kosher designation could not have been too costly, especially given the free press they earned, directed squarely at their target patients. Pfizer improved the experience for this patient segment, who had the satisfaction of knowing their drug is working to make them not just healthier, but happier.
Understanding Pain Points—And Putting Yourself in Patient’s Shoes
Pharmaceutical companies—and the consultants who serve them—spend countless effort understanding patient pathways by identifying pain points, linkages, and roadblocks. Marketers spend entire careers attempting to put themselves in patients’ positions.
Today, these insights are used primarily to drive sales, but they are certainly not incongruent with the Triple Aim. As Otsuka, Vertex, and Pfizer demonstrate, what’s right for patients is equally beneficial to the health system. And while the cases may seem opportunistic, they responded well to the target patients and bolstered a position in a given market at a specific point in time. Not all drugs need a mobile app or can be reformulated with smart technology; many prescriptions are too simple to benefit from intricate packaging; and it’s unlikely that the Kosher union will certify more than a handful of drugs in the next century. But in each case, outside-the-box marketing teams empowered their patients to take ownership of their own treatments, which is exactly what must occur to transform the Triple Aim from idea to reality, regardless of what regulations come pharma’s way.
Note: When Pfizer launched Elelyso in 2012, they entered a crowded field that had been led by a clear leader for nearly two decades. Pfizer’s product came third to market and was not clinically differentiated. Reimbursement was seldom an issue, so the team couldn’t compete solely on cost. Instead, they sought to appeal to their community in less traditional ways.




