Therapeutic Talk: Finding a Powerful Antifungal Without the Toxicity
Amphotericin B is an antifungal that not only kills many of the most dangerous fungi to humans, but it also remains powerful even as bacteria and fungi continue to build resistance against many of the other antibacterials we use for treatment today. The downside to this potent drug is it can be quite toxic and poses risks to the human body even in small doses. A standard dose of the medicine causes acute kidney damage in 20% of patients and 50% of those who receive higher dosages used to treat life-threatening infections on a broad spectrum.
However, researchers at the University of Illinois Urbana-Champaign and the University of Wisconsin-Madison have found a compound that they call AM-2-19 that can reportedly treat severe systemic fungal infections in mice without harming the kidneys or losing the efficacy of true amphotericin B. The researchers have developed candidate SF001 from the compound and have moved it into Phase 1 clinical trial under biotech Sfunga Therapeutics.
“I think this is going to be a very important product and anti-fungal,” stated Kieren Marr, MD, Co-founder and Chief Medical Officer at Sfunga.
Brand Beat: Visualizing Hearing Loss

What does hearing loss look like? For people who have never experienced hearing loss, answering this question can unlock a better understanding of what hearing loss is really like.
The Denmark-based hearing device company GN is partnering with Soundly, a consumer education resource for hearing wellness, to develop a campaign to demystify hearing loss and hearing health called “Inside the Ear.” This campaign contains a series of videos with animated visuals by Design Cells which beautifully illustrate the complex inner workings of the ear.
The partnership is aiming to spread awareness of the crucial significance of maintaining optimal hearing health. The engaging videos capture different elements of the ear and hearing function to foster a deeper understanding of how ears work.
“At GN, our mission extends beyond providing innovative hearing technologies. Through campaigns such as ‘Inside the Ear’ we are dedicated to educating and empowering individuals, enabling them to make informed decisions about their hearing health and inspire proactive steps toward achieving better hearing,” explains Mirjam van Oort-Lohuis, Chief Marketing Officer, GN Hearing.
Some commonly unknown topics touched upon are how sound reaches the brain, what noise-induced hearing damage looks like, and how tinnitus really affects the ear. The videos can be viewed at www.gn.com/inside-the-ear.
Discoveries and Innovation: Revolutionizing Brain Tumor Detection
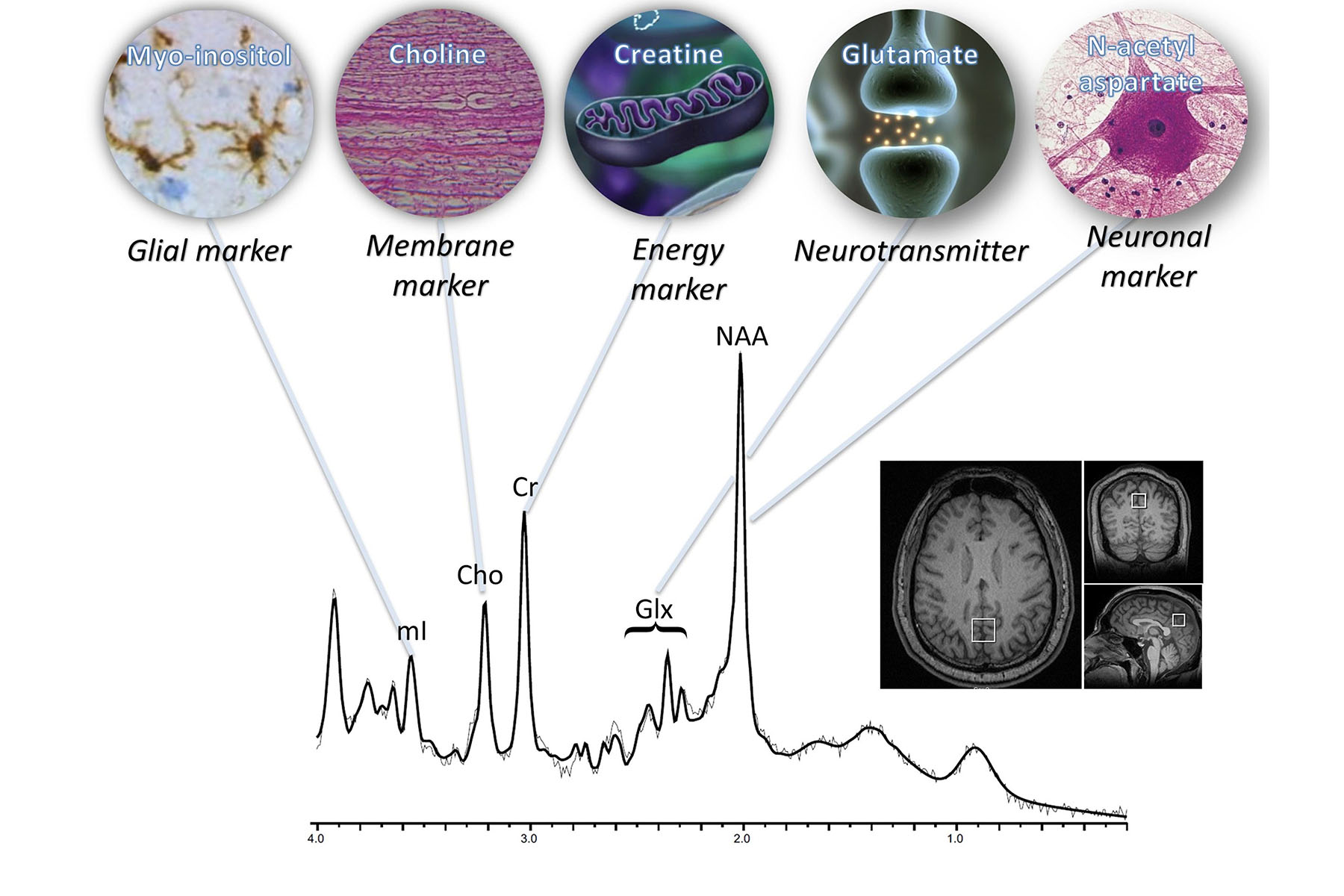
BrainSpec is a software program that enables virtual biopsies of the brain. The innovative company received FDA approval for its Magnetic Resonance Spectroscopy (MRS) platform, BrainSpec Core, to provide non-invasive measurement of brain chemistry to support the diagnostic process in the most common brain-related illnesses, including Alzheimer’s disease, multiple sclerosis, epilepsy, brain tumors, and more.
BrainSpec Core uses non-invasive imagery from standard MRI machines to measure concentrations of chemicals in the brain in order to create a virtual biopsy. While standard MRI produces images of the brain by visualizing fat and water distributions, MRS detects metabolites across the brain and over time–generating multidimensional data about the chemical composition of tissues that can be fed into AI models. The software is compatible with MRI machines, allowing it to be accessed by any medical facility via cloud-based systems.
“There are several key advantages of this AI solution that have important clinical relevance,” said Raymond Y. Huang, MD, PhD, Division Chief, Neuroradiology, Brigham and Women’s Hospital. “First, it provides quantitative neurometabolite measurements that can be used to aid in monitoring the changes in brain tumors that are undergoing therapy. The second strength is that it automates the delivery of the MR spectroscopy results, reducing turn-around time from days to minutes which is critical for the clinical care of patients. Finally, ability to measure 2-hydroxyglutarate and other brain metabolites provides a direct and non-invasive marker of low-grade gliomas that could lead to a more accurate classification of individual tumors benefiting decision making.”
According to BrainSpec, adding MRS to the diagnostic process has been shown to save as much as $98K per patient in healthcare costs compared to current standard of care alone over a five-year time period. The cutting-edge tools can help physicians make more precise diagnosis and treatment decisions with information that has been previously unavailable, including measurements for relative 2-hydroxyglutarate levels, an oncometabolite present in low-grade gliomas. Making spectroscopy more available to the public leads to better visualization of individual tumors, which can lead to better diagnostics and ultimately benefit brain tumor patients.
FDA Update
Drug Approvals
Ogsiveo (nirogacestat) is the first drug to be approved by the FDA for the treatment of patients with desmoid tumors, a rare subtype of soft tissue sarcomas. The tablets are created by SpringWorks Therapeutics Inc. for adult patients with progressing desmoid tumors who require systemic treatment.
Valneva received FDA approval for IXCHIQ, a vaccine against the chikungunya virus. This is the first vaccine for the vector-borne disease to receive marketing approval. Valneva’s proprietary Recombumin is a human and animal origin-free recombinant human albumin that serves as a main enabler for the vaccine formulation.
Eli Lilly’s Jaypirca (pirtobrutinib) was approved for the treatment of adult patients with chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL/SLL) who have received at least two prior lines of therapy, including a Bruton’s tyrosine kinase (BTK) inhibitor and a BCL-2 inhibitor.
Med Device Approvals
Alafair Biosciences received 510(k) clearance for VersaWrap to be used in all populations, regardless of age. Younger patients can now benefit from VersaWrap, a class II medical device that is a bioresorbable hydrogel implant. Implanted during orthopedic surgeries, the wrap forms a gelatinous layer around target tissues such as tendons, peripheral nerve, and surrounding soft tissues, allowing gliding during healing and reducing postoperative tethering to give consistent results.
FDA granted 4DMedical clearance for its computed tomography (CT) based ventilation product CT LVAS. The product joins 4D Medical’s XV LVAS imaging software cleared for use with fluoroscopy in the United States. CT LVAS provides HCPs with additional data on lung performance along with the structural detail of CT scans. This capability fills an information gap that enables radiologists to provide the objective, data-driven information that could materially inform diagnosis and treatment options.







