Patient Pages: Patients More Concerned for Loved Ones
A recent national survey by Astellas Pharma US, Inc. and Medivation, Inc. suggests that U.S. men with advanced prostate cancer are more concerned with burdening loved ones than with dying. Patients and caregivers were both subjects of the Advanced Prostate Cancer Patient and Caregiver Burden of Illness Survey.
Of the men with cancer:
- 45% report that they remain silent about their cancer and the treatments involved.
- 59% say they’re worried about becoming a burden to family and friends.
- 43% have the same level of concern for death.
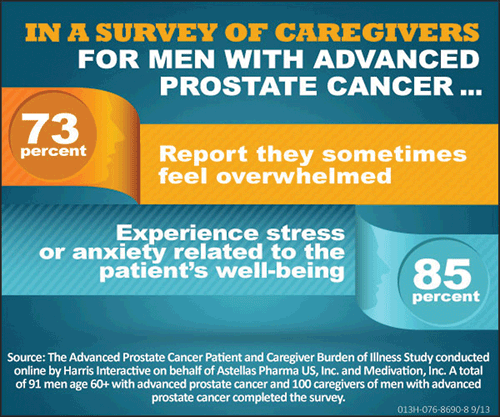
The caregivers surveyed showed:
- 73% admit to feeling overwhelmed by caring for someone with advanced prostate cancer.
- 85% say they experience stress and anxiety for their loved one’s well-being.
- 83% respond with concern for helping their loved one cope with the physical and mental effects of prostate cancer.
Astellas and Medivation commissioned the survey through Harris Interactive. Four leading cancer organizations sponsored the survey.
DC Dispatch: ER Reimbursement Should Rise Under the ACA
Due to the high rate of the uninsured in the United States, ERs have suffered shortfalls in reimbursement for decades. When the Affordable Care Act is fully implemented, emergency departments may begin receiving “considerably more” reimbursement. Annals of Emergency Medicine recently published a study that showed how emergency department reimbursements could improve.
“Assuming typical reimbursement patterns continue, emergency department reimbursement for outpatient visits to the ER may increase by 17% for uninsured people who go on Medicaid and by 39% for uninsured people who move to the private insurance market,” says lead study author Jessica Galarraga, MD, MPH, of the Department of Emergency Medicine at George Washington University.
Therapeutic Talk: Atrial Fibrillation Linked to Stroke
More than 500,000 people have shown support for the Sign Against Stroke in Atrial Fibrillation (AF) campaign, which calls for action against the thousands of preventable strokes that kill or disable many AF patients every year.
Approximately 15 million people experience a stroke every year worldwide. About one-third of these suffer permanent disabilities and another third die, accounting for 10% of all deaths globally. Sign Against Stroke in Atrial Fibrillation supports the world’s only Global AF Patient Charter that calls for more awareness of the signs of AF and AF-related stroke. For more info, visit www.signagainststroke.
Sales Sector: Paradigm Shift in Access
Decision Resources Group reports a paradigm shift in the way pharmaceutical companies and brands gain access to the U.S. market. What’s causing the shift? Accountable care organizations (ACOs) are continuing to develop across the U.S. (more than 500 now exist) and enrollment on the health exchanges moves forward.
Comprehensive surveys of physicians and ACO executives show the enormous changes in the U.S. healthcare market that are affecting pharmaceutical brands. Looking at currently available and emerging brands by therapy, surveys show some will benefit and others will be challenged.
For instance, for schizophrenia and depression, physician and payer responses suggest constrained use of branded therapies on exchange-based plans because of generic competition—even though they feel Abilify and Cymbalta will remain popular therapies. In light of the new dynamics shaping reimbursement decisions, pharmaceutical brand teams will craft market access strategies that take these changes into account.
Discoveries/Innovations: Alere Announces New HIV Test
Alere, Inc., a leading provider of point-of-care rapid diagnostic and health information solutions, just announced the immediate U.S. availability of the Alere Determine HIV-1/2 Ag/Ab Combo test, which is the first FDA approved rapid point-of-care test that detects HIV-1/2 antibodies and the HIV-1 p24 antigen.
The availability of the Alere Determine Combo test will contribute measurably to public health by helping HIV-positive individuals to become aware of their HIV status earlier, thereby potentially reducing HIV transmission, according to the company. The test has been FDA approved to be sold in the U.S. as a Clinical Laboratory Improvement Amendments (CLIA) device.
Doctor Docs: ZocDoc’s Digital Doctor Survey Results
The results from ZocDoc’s first annual Digital Doctor Survey are in. The 2013 survey explored the use of online reviews, practice management, and social media among healthcare providers including primary care doctors, dentists and cardiologists.
The survey shows that 85% of respondents monitor online reviews about themselves, and that 36% look at competitor’s reviews. Also:
- 23% surveyed said that they think online reviews are very fair.
- 62% believe they are fair.
- 15% said that they are not fair.
Additionally, ZocDoc found that 53% of physicians surveyed have a Facebook account for their practice, 34% use Google+, 28% use LinkedIn and 21% have a Twitter account.
FDA Update
FDA Approves Bluetooth Technology
Nonin Medical, Inc., inventor of finger pulse oximetry and a leader in noninvasive medical monitoring, announced that the FDA has cleared the Nonin Model 3230 Bluetooth Smart finger pulse oximeter for use in the U.S. The oximeter is one of the first medical devices to incorporate Bluetooth Smart wireless technology, which helps to facilitate simple and secure connections to Bluetooth Smart ready devices for vital information exchange over a secure wireless connection.
Inhibitor Recommended for Hepatitis C
Janssen Research & Development, LLC has announced that the Antiviral Drugs Advisory Committee of the FDA voted unanimously (19 to 0) to recommend approval of the investigational protease inhibitor, simeprevir (TMC435). The inhibitor would be administered once daily as a 150 mg capsule with pegylated interferon and ribavirin for the treatment of hepatitis C in adult patients with compensated liver disease.
New FDA Grants
The FDA recently awarded $14 million in grants for the development of treatment for rare diseases. Awards were given to:
- Leonide Saad, Alkeus Pharmaceuticals, Inc., for the Phase 1 Study of ALK001 for the treatment of Stargardt Disease.
- Parinda Mehta, Children’s Hospital Medical Center Cincinnati, for the Phase 1 Study of Quercetin for the treatment of Fanconi Anemia.
- Diva De Leon, Children’s Hospital of Philadelphia, for the Phase 2A Study of Exendin for the treatment of Congenital Hyperinsulinism.
- Soma Jyonouchi, Children’s Hospital of Philadelphia, for the Phase 1 Study of IL-2 for the treatment of Wiskott-Aldrich Syndrome.
- Dwight Koeberl, Duke University, for the Phase 1/2 Study of Clenbuterol for the treatment of Pompe Disease.
The FDA’s Orphan Products Grants Program was created by the Orphan Drug Act to promote the development of products for rare diseases.