Doctor Docs: Daily Aspirin Treatment for Older Adults Refuted
For years, doctors have recommended taking a low dose aspirin once a day to older adult patients trying to reduce their risk of heart attack. New guidelines from the American College of Cardiology and the American Heart Association say this is no longer recommended as a preventative measure for the elderly patients who don’t have a high risk or existing heart disease.
This approach may still work for certain older high-risk patients, such as those who have trouble lowering their cholesterol or managing their blood sugars, but they should not be at a higher risk for internal bleeding while taking aspirin. As for younger adults, the anti-clotting drug is now a much lower ranked recommendation due to the lack of definitive research and much controversy over its effects.
“Clinicians should be very selective in prescribing aspirin for people without known cardiovascular disease,” John Hopkins cardiologist Dr. Roger Blumenthal, who co-chaired the new guidelines, said in a statement. “It’s much more important to optimize lifestyle habits and control blood pressure and cholesterol as opposed to recommending aspirin.” This is simply because, for those without a known heart condition, taking aspirin could have no effect or increase risk of internal bleeding and early death without any positive effects. Doctors must closely consider a patient’s individual risk of bleeding, which could be affected by anything from kidney disease, heart disease, and diabetes to high blood pressure and old age. Dr. Blumenthal summarizes, “Aspirin should be limited to people at the highest risk of cardiovascular disease and a very low risk of bleeding.”
Therapeutic Talk: Combating Postpartum Depression

The FDA approved the first and only drug that specifically treats postpartum depression (PPD). Unlike traditional antidepressants, Zulresso is delivered intravenously over 60 hours and works rapidly. Sage Therapeutics developed the drug as the first to exclusively treat postpartum depression, the most common complication of childbirth experienced by one in nine American women who suffer sadness, anxiety, irritability, withdrawal from friends or family, trouble bonding with their babies, and thoughts about harming themselves or more rarely, their babies.
“We are proud to be a part of this important moment in mental health, creating the opportunity for an unprecedented change in the way postpartum depression is thought about and treated moving forward,” Jeff Jonas, MD, CEO of Sage, said in a statement. “We are grateful for the patients, researchers, healthcare providers, advocates, caregivers and Sage employees who helped secure the approval of the first medicine specifically for postpartum depression. We believe Zulresso will address an important need for women’s mental health. The impact of PPD is multi-generational, and we look forward to bringing Zulresso to patients in urgent need of a new treatment option. We believe Zulresso will be a catalyst in starting a new dialogue emphasizing the importance of women’s mental health, and the importance of diagnosing and treating PPD.” The new drug is expected to be available this June through a restricted program called the Zulresso REMS Program that requires the drug be administered by a healthcare provider in a certified healthcare facility.
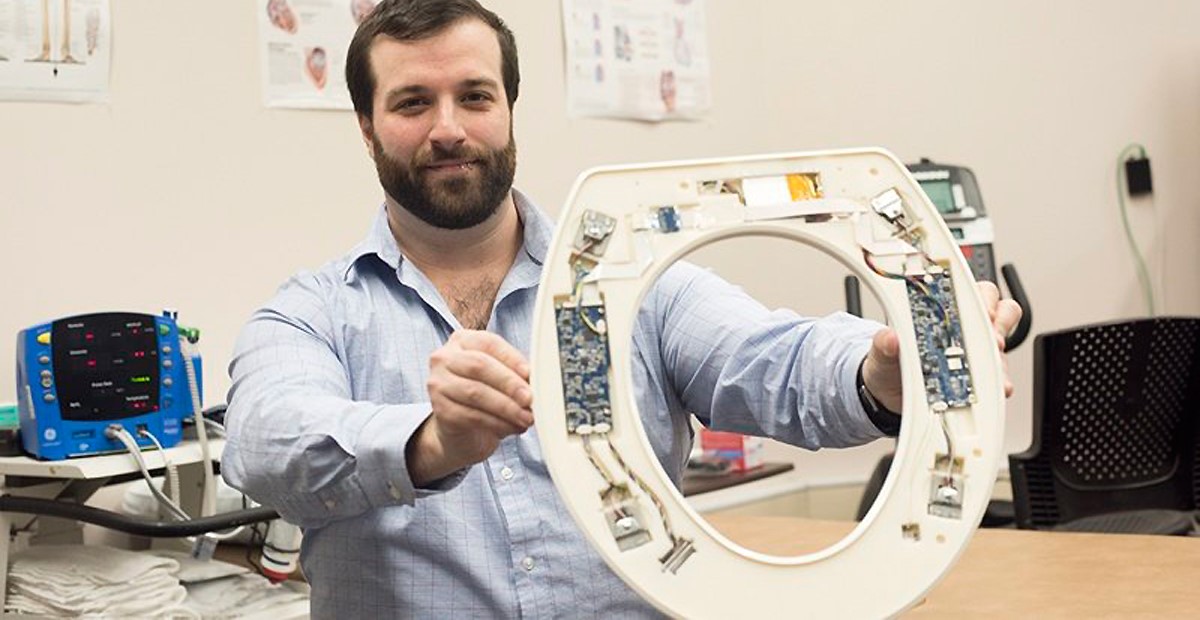
Medical Device: Smart Toilet Seats Detect Heart Failure
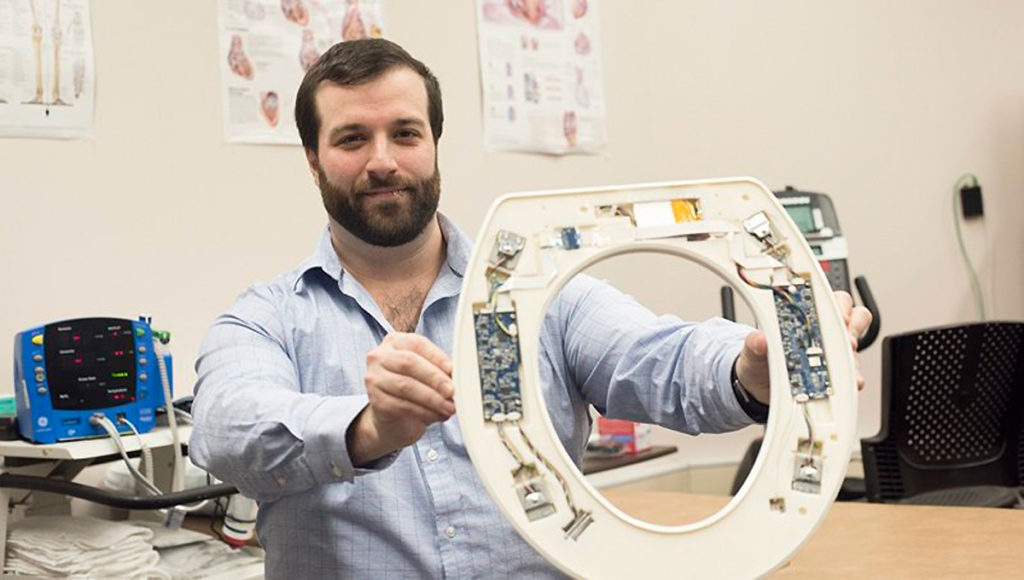
A smart toilet seat has been developed to spot the signs of congestive heart failure right at home. Researchers at the Rochester Institute of Technology’s Heart Health Intelligence Program created the device with the idea that it would lower hospital admission rates for patients recovering from a heart attack, heart failure, or coronary artery bypass surgery, as well as those with pneumonia, chronic obstructive pulmonary disease, and total hip or knee replacements. They aimed to design an FDA approved device that could be given to such patients as they check out from the hospital. “Typically, within 30 days of hospital discharge, 25% of patients with congestive heart failure are readmitted,” said Heart Health Intelligence’s Founder and CEO, Nicholas Conn, PhD, a postdoctoral fellow at RIT and a member of the development team, in a statement. “After 90 days of hospital discharge, 45% of patients are readmitted.”
Sensors within the high-tech toilet seats can record a person’s heart rate, blood pressure, and oxygenation levels, as well as the amount of blood pumped out of the heart with every beat. Using this data in conjunction with data from regular check-ins, the company hopes to alert healthcare providers of worsening conditions before symptoms appear so they can provide less-costly interventions. Dr. Conn estimates the loss for one hospital readmitting 150 patients can top about $500,000 per year, while providing 150 of the company’s smart toilet seats would cost the hospital $200,000.
Trend Setting: Approaching Heart Failure Through Fat Metabolism
A compound crucial to metabolizing fat has been found to be severely diminished in patients whose hearts are stressed. Scientists at Ohio State University believe that by restoring this compound, the body can continue burning fats and glucose that the heart needs for the energy necessary to function well. This would help many patients avoid heart failure.
Doug Lewandowski, PhD, an Ohio State researcher who focuses on cardiac metabolism, says that “before any physical signs or symptoms of heart failure emerge, chronically stressed cardiac cells undergo changes to adapt, but the adjustments don’t necessarily make things better.” The deficiencies in acyl-CoA that result from stress on the heart actually disrupt its energy production cycle, leading to toxic fat accumulation that impairs the heart’s ability to function and pump properly.
The scientists tested human heart tissue samples before and after receiving a left ventricular assist device. They found that the post-LVAD samples had recovered normal levels of acyl-CoA, since the heart doesn’t have to work as hard with the help of the pump. Maintaining acyl-CoA allowed the hearts to burn fat and generate energy, raising hopes for disease mitigation.
Patient Pages: HCPs Could Do More for Their Autoimmune Patients
Recent surveys reveal that while cancer patients feel satisfied with their oncologists, autoimmune disease patients are finding their primary healthcare providers lacking. According to Health Union’s 2018 HCP Satisfaction Survey, more than eight in 10 cancer patients reported an overall high level of satisfaction with their HCPs and the control of their condition with their treatment plan, while six in 10 patients with autoimmune conditions felt the same way about their regular providers. This response is partially attributed to the perceived effectiveness of the treatment plan on the patient’s symptoms and overall condition. Almost seven in 10 cancer patients reported that their conditions feel under control on their current treatment plans, while less than a third of autoimmune patients felt this way.
The surveyed cancer patients find their doctors to be more personable and attentive during appointments, and concerned about their patient’s mental health. Meanwhile, the survey reveals a gap in the care of autoimmune patients who are much less likely to recommend their own providers due to their inability to answer questions, review test results, communicate effectively, or clearly explain how and when to take medications.
“Although the level of care and thoroughness doctors show patients with cancer is encouraging and shows an evolution toward a better understanding of patient needs, it’s unnerving to see that people living with other conditions don’t feel they receive a similar level of attention,” said Tim Armand, Co-founder and President of Health Union. “From interactions occurring within our communities, we know that HCP relationships can positively impact patients, regardless of their condition.” Health Union believes that a major factor that might explain higher HCP satisfaction among cancer patients is the additional services frequently offered by their doctors, such as on-call staff on weekends and evenings, online portals to ask questions, and access to test results. Patients with autoimmune conditions were less likely to indicate their HCPs offered these services, revealing an underserved market in this community.
FDA Update
Drug Approvals
The FDA approved Pfizer’s Trazimera, an oncology biosimilar to Herceptin. The IV drug treats human epidermal growth factor receptor-2 (HER2) overexpressing breast cancer and HER2 overexpressing metastatic gastric or gastroesophageal junction adenocarcinoma. Trazimera targets HER2, a protein found on the surface of some cancer cells which can stimulate the cells to divide and grow. The drug binds to the HER2 protein and blocks the receptors, stopping cell division and growth.
A new generic version of Diovan (valsartan) has been approved to treat high blood pressure and heart failure. The angiotensin II receptor blocker (ARB), manufactured by Alkem Laboratories Limited, was prioritized in order to close the gap from a recent shortage of this critical medicine due to multiple recalls of generic valsartan products from several manufacturers.
Med Device Approvals
Medtronic’s Resolute Integrity Zotarolimus Eluting Coronary Stent System has been approved as a drug-coated stent and the catheter delivery system with a balloon that inflates to deploy the stent. The stent is placed into a coronary artery to supply blood to the heart and keep the coronary artery open.
OPKO Diagnostics’ Sangia Total PSA Test has been approved by the FDA to market as an aid in the diagnosis of prostate cancer. The Cassette Assembly and Sample Collector system is the first point of care test that can deliver PSA results near where the patient is being evaluated, for example at the doctor’s office, instead of waiting for lab analysis. The test can be administered with a simple finger prick, and results are generated in less than 15 minutes.