Medable Inc. announced the launch of The ACCESS initiative—short for American COVID-19 Collaborative Enabling Seamless Science. This collaborative effort, which includes Medable as well as BioIntelliSense, Datavant, Parexel, PWNHealth, and the American Heart Association’s Center for Health Technology and Innovation, was formed with the aim to accelerate development of diagnostics and treatments for COVID-19. The initiative provides a mobile consumer application (only available on Apple at launch) and secure infrastructure to quickly connect health researchers and clinical trial teams securely with up to millions of home-bound individuals in the United States.
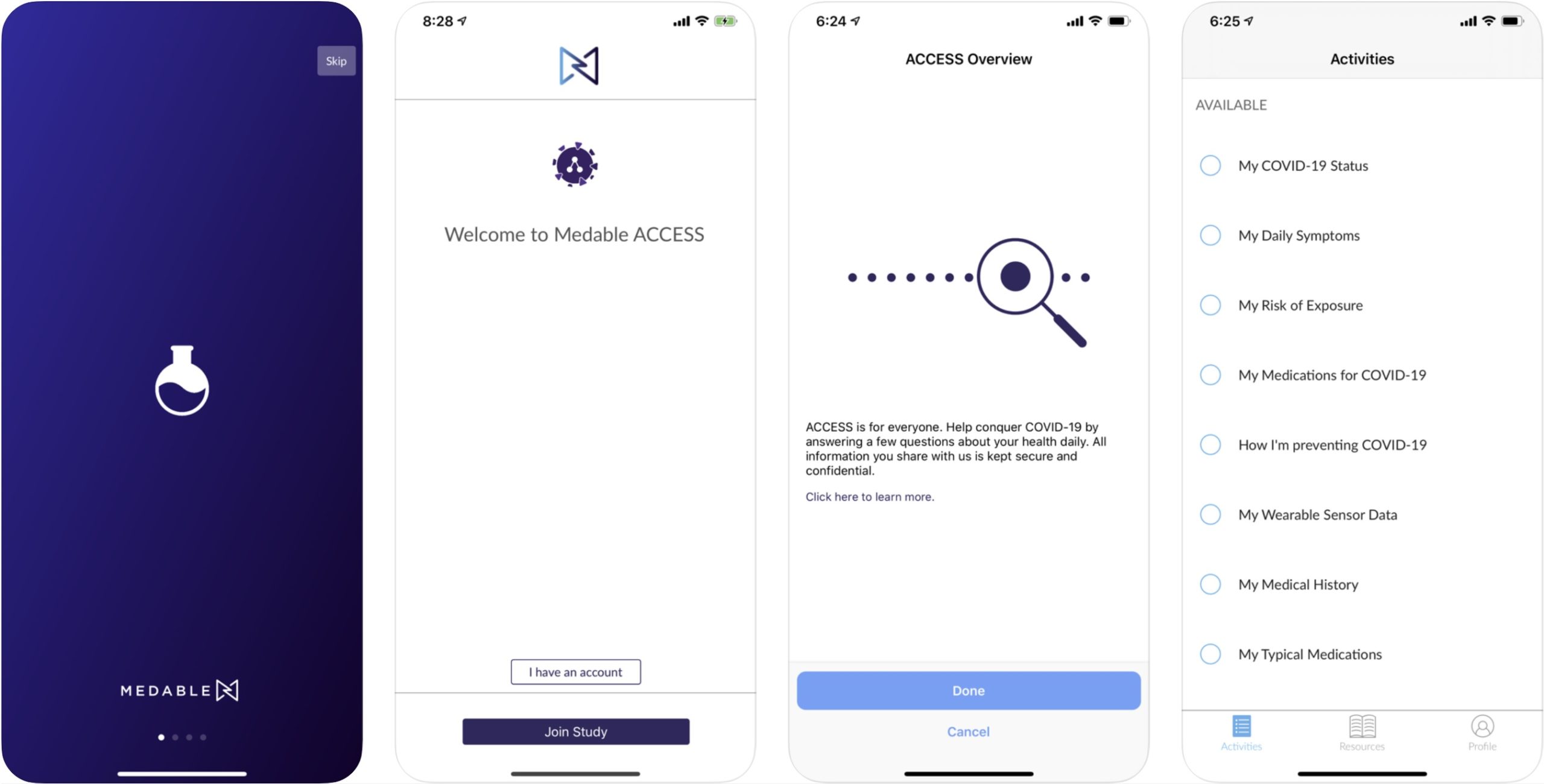 Medable built the mobile application and digital infrastructure for ACCESS, which will make it easy for individuals to contribute specific information about their COVID-19 experience, combine it with health records and data from wearable devices, and opt in to participate in current and future studies for diagnostics, treatments, and vaccines. The data that people share can be quickly and anonymously matched to research studies, providing researchers with a foundational framework for dynamic research at scale. Participants opt in at every stage, so they maintain control over their personal health data—and decide how they want to engage in potential studies.
Medable built the mobile application and digital infrastructure for ACCESS, which will make it easy for individuals to contribute specific information about their COVID-19 experience, combine it with health records and data from wearable devices, and opt in to participate in current and future studies for diagnostics, treatments, and vaccines. The data that people share can be quickly and anonymously matched to research studies, providing researchers with a foundational framework for dynamic research at scale. Participants opt in at every stage, so they maintain control over their personal health data—and decide how they want to engage in potential studies.
“ACCESS will enable us all to accelerate diagnostic testing and clinical trials—and advance important monitoring and immunity research—so that we can conquer COVID-19 with effective prevention and intervention strategies,” Dr. Michelle Longmire, CEO and Co-founder of Medable, said in a statement. “By empowering people in their homes with ACCESS, we can accelerate research by reducing time for enrollment and data collection.”
Furthermore, ACCESS takes full advantage of mobile and digital health technologies to facilitate at-home research, clinical trial access, and population-based long-term outcome studies. The infrastructure combines medical-grade wearable sensors, patient-reported data and outcomes, historical health data, and health record aggregation.
For example, ACCESS provides screening, disease and immunity status testing, and general lab testing to qualified participants through PWNHealth’s national network. And the initiative enables vital sign data collection via smart watches including the Apple Watch, as well as continuous health monitoring through FDA-cleared body-worn sensors from BioIntelliSense. Meanwhile, Datavant’s data infrastructure enables participants to provide researchers access to de-identified health records, claims, diagnostic, and other data sources. And Parexel will offer their expertise and services as a global contract research organization (CRO).
“We have seen a rapid response from the pharmaceutical industry to the COVID-19 crisis, with hundreds of clinical trials underway in a remarkably short period of time,” Jason Martin, Senior Vice President, Global Data Operations, Parexel, said in a statement. “The next challenge is connecting patients to these studies, which is particularly difficult in this fast-changing situation. Fortunately, today we have access to more data as well as innovative ways to de-identify and link that data to help connect the right patients to the right studies. We look forward to working with the ACCESS study team to harness that connectivity to bring hope to patients in need.”
The American Heart Association’s role was in part to help Medable bring various parties together to contribute to ACCESS.
“Connecting innovative companies in health tech is a big part of what we do at the Center,” Patrick Wayte, SVP, American Heart Association Center for Health Technology and Innovation, said in a statement. “Convening parties to develop novel ways of improving clinical care and research, with the ultimate aim of improving patient outcomes, is a core philosophy of the Association. We see that digital healthcare solutions can play a huge role in the transformation of medicine, and we’re glad to help catalyze these changes.”
More companies can join the collaborative by visiting https://access.medable.com/ to learn more or contacting access@medable.com.