Unlike common illnesses, the general public and even the healthcare community have little awareness about rare diseases. With low incidence rates, the lack of ICD-10 designation, and limited therapies, primary care physicians have a poor understanding of disease states, manifestations, and interception symptomology. In fact, most of the information they receive on rare diseases is provided by those afflicted with the diseases sharing information via blogs, advocacy sites, or registries that are not medically vetted.
Additionally, the genetic variability of many rare diseases results in highly variable presentations, which leads to a protracted odyssey of frequent non-diagnosis, underdiagnosis, or misdiagnosis. Even when patients are properly diagnosed after suffering the distress of multiple years of uncertainty, therapies tend to be prohibitively expensive and administered through tertiary treatment centers or academic research institutes that cover large geographies, creating a significant financial and travel burden.
The average peak U.S. revenue of therapies launched over the past 15 years is about $800M, with only one in five reaching U.S. sales of $1B, and over half failing to reach peak U.S. sales of $250M.
Established pharmaceutical companies with limited experience in rare disease are increasingly looking to commercialize therapies for these uncommon conditions. In addition, there is growing interest in early-stage companies using the rare disease pathway as a step to become fully integrated biopharmaceutical companies. But while these organizations have expertise in the research and development of rare disease indications, most lack the insight and experience in launching a drug that treats these illnesses. For these companies, it is important to master and refine a real-world playbook to achieve commercial success.
The Vast Differences in Launching Rare Disease Drugs
The launch of a drug is complex, impacts financial performance beyond initial market entry, and often fails to meet pre-sales expectations. For common and well-understood diseases with existing diagnostic codes, treatment options, and a highly educated primary care physician community, the healthcare ecosystem can support a successful launch, as many patient needs are already met. And traditional DTC and NPI-based programmatic, social media, and television advertising are commonly utilized options for this phase. However, with rare diseases, the landscape is dramatically different.
On average, therapies reach ~50% of peak sales by year three, and these early-year sales are strongly predictive of ultimate peak sales.
In many cases, the biopharma company must develop a deep understanding of the unmet or unknown need of patients and healthcare providers before identifying ways to build the strategy, infrastructure, and programs needed for launch success. These must be grounded in uncovering the ideal patient population, mapping their diagnostic and treatment journey, and linking their healthcare ecosystem, whether primary care physicians, bona-fide specialty care physicians, or inferred specialty care providers.
For common illnesses, patients transition from disease awareness and recognition to presentation and diagnosis. Therapeutic options are considered, a treatment course is selected, and a brand best suited to known needs is chosen. The patient then secures the therapy and adheres to the regimen designed to achieve improvements in their health. The patient population, healthcare journey, and supporting physicians are easily uncovered through the analysis of highly available insurance claims and prescription drug data. But this same analysis for a rare disease will be fraught with significant numbers of missing patients, pronounced gaps along the diagnostic and treatment journey, as well as incomplete linkages with treating physicians.
About half of all products launched over the past 15 years have underperformed pre-launch consensus forecasts by more than 20%.
A System Designed to Improve the Launch Process
Enter a System of Insight, one that utilizes Machine Learning and Artificial Intelligence in conjunction with a curated pool of smart, privacy-safe Real-World Data. This system and its unification of people, process, technology, and data, is indispensable in creating a high-definition, intelligence-driven enterprise equipped to make smarter product launch decisions.
Simply, the System of Insight can find patients with a high propensity of disease affliction, map their healthcare pathways, and identify treating physicians—even those who are “hidden” because they are not formally designated as relevant specialty care providers.
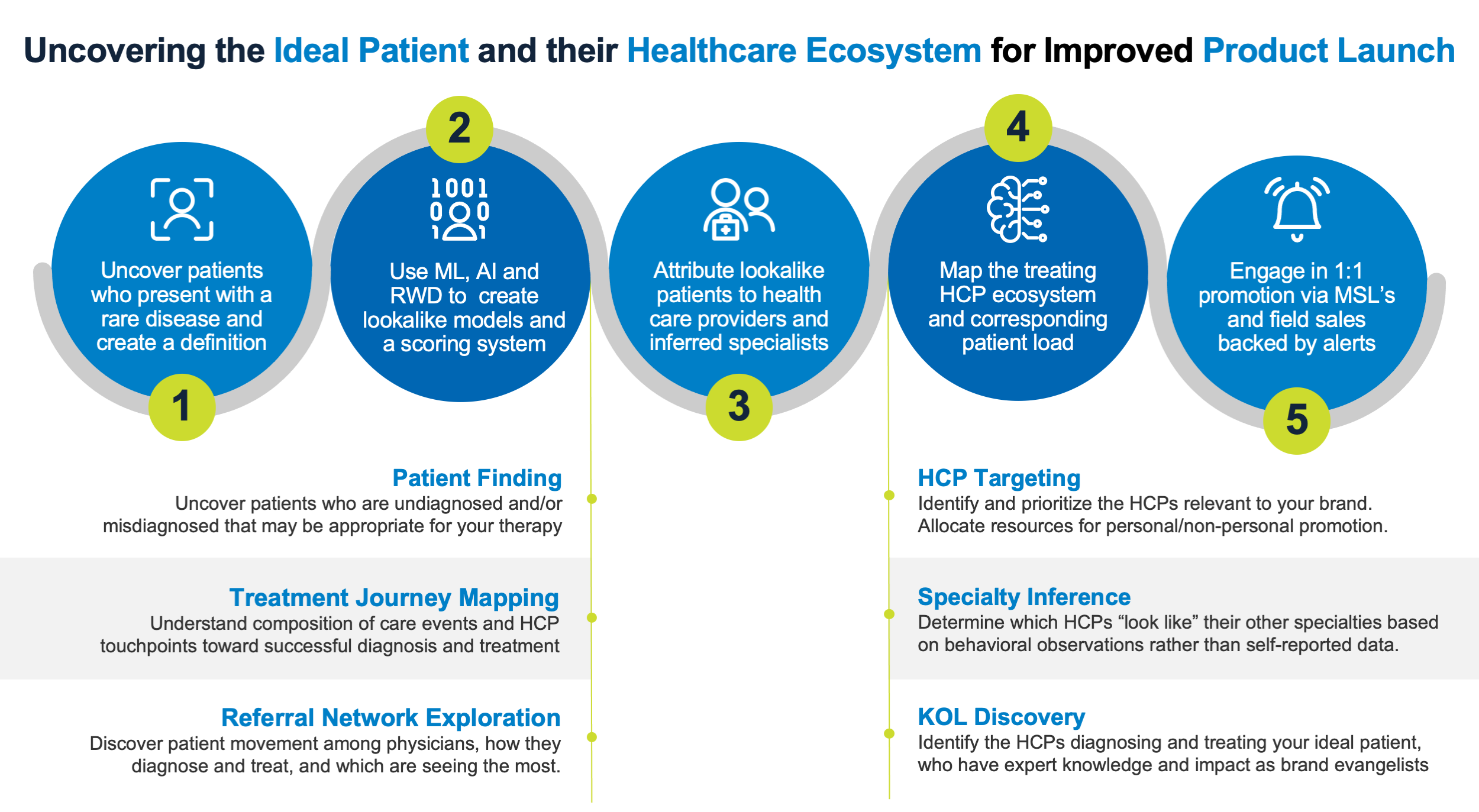 The goal of a product launch as well as follow-on commercial activities is to ensure the greatest number of candidates have access to a treatment, fully realize patient lifetime value, and maximize shareholder return on investment. Establishing a therapy as the standard of care and thus creating a trajectory towards peak sales can now be more easily accomplished by using a System of Insight.
The goal of a product launch as well as follow-on commercial activities is to ensure the greatest number of candidates have access to a treatment, fully realize patient lifetime value, and maximize shareholder return on investment. Establishing a therapy as the standard of care and thus creating a trajectory towards peak sales can now be more easily accomplished by using a System of Insight.
This System of Insight allows companies to provide the actionable intelligence necessary to personally engage with HCPs treating bona-fide, high-value candidates for a therapy as well as provide disease interception and intervention education. By building deep relationships with these HCPs and assisting in the diagnosis and treatment of high-value patients—most of whom trust the opinions of their healthcare providers over programmatic, social, and television advertising—life sciences organizations can now be better equipped for launch success.







