Good science dictates we make investments in diversity and inclusion to create representative populations in our clinical trials, but there is still room to improve.
Clinical trials have historically underrepresented certain populations. But now that the Food and Drug Omnibus Reform Act (FDORA) is law,1 sponsors of most trials must submit diversity action plans to the agency, report demographic data for participants, and actively recruit from marginalized populations, which is helping to make diversity a greater priority across trial portfolios.
In March 2023, Bristol Myers Squibb announced a multimillion dollar investment2 in its global inclusion goals, including a focus on increasing clinical trial diversity. The company claims that nearly 60% of its clinical trial sites are now located in racially and ethnically diverse areas.
Other federal agencies also support this effort. Advanced Research Projects Agency for Health (ARPA-H) announced3 an initiative in October 2023 to diversify and improve access to clinical trials. The U.S. Department of Health and Human Services (HHS) recently invested $2.5 billion in federal research funding to address pressing diversity issues. And the Advancing Clinical Trial Readiness (ACTR)’s initiative aims to enable 90% of all eligible Americans4 to participate in a clinical trial within a half hour of their home.
While the industry is moving in the right direction, racial and ethnic minorities still only account for 2% to 16% of clinical trial participants5 while comprising 39% of the U.S. population. One big obstacle: patient recruitment and retention from historically underrepresented populations. Sponsors are now starting to collaborate with specialty partners to carefully plan and execute new strategies that chip away at traditional participation barriers and are more conducive to reaching broader, more representative demographics.
5 Ways to Reach 2024 Clinical Trial Diversity Milestones
The diversity challenge will not be solved simply by recruiting more of a sub-population—this misses the point. The New England Journal of Medicine (NEJM) recently published6 the following requirement for papers submitted for publication, stating: “…we will require, as a new step, that authors of research studies prepare a supplementary table that provides background information on the disease, problem, or condition and the representativeness of the study group, to be posted with the article at the time of publication.”
The key phrase, “representativeness of the study group,” is also what global regulatory bodies seek via their latest guidance documents. Fundamentally, a representative patient sample in a clinical trial is good science, while diversity just for diversity’s sake has nothing to do with science—and this may be tripping up sponsors. A representative patient sample should always be the guiding principle. But because it often wasn’t a focus in the past, many other factors have gotten in the way of research’s most prudent prerequisite. These factors include cost, speed, bias, infrastructure failures, patient mistrust, and participation burdens.
To overcome these issues, here are five actions all stakeholders can take to move the needle in population representation in clinical trials this year.
1. Leverage Alternative
Tech-Enabled Clinical Trial Models According to the Advarra 2023 Study Activation Survey Report,7 35% of sites and 33% of sponsors already employ alternative trial designs in at least some trials. And, EY-Parthenon survey8 respondents say that by 2024, half of all clinical trials will be hybrid or remote.
Alternative study designs and modalities, such as decentralized clinical trials and eConsent, can help expand a trial’s reach. For example, a study incorporating telehealth tools and at-home study visits can reach more people than a traditional trial taking place solely at the site’s physical location. Another example is providing wearable sensors to trial participants, such as in a sleep apnea trial9 where the sponsor provided Withings’ Sleep Tracking Mats that patients used at home rather than requiring frequent trips into a lab. Not everyone can (nor wants to) be at a site during business hours or has reliable transportation—especially as the site may be far from home.
“We can improve diversity in clinical trials with a smart, intentional combination of touch plus tech through both in-person and digital community engagement,” said Camille Pope, Chief Medical Lead at Acclinate, a health technology company specializing in health equity through inclusive research. “Trust-enabling technology is crucial to broaden our reach and can enhance in-person relationship building.”
Connecting with potential trial participants in the community where they receive regular healthcare can also help reach more populations. We must look beyond major academic medical centers, especially as many lower income patients are increasingly leaving big cities, making transportation difficult. Research professionals should consider how such alternative approaches and technologies can improve recruitment and enrollment efforts.
2. Partner With Diverse Community Groups and Leaders
It’s important to build relationships with diverse community groups, but that takes time and consistency. In populations historically distrustful of clinical research, organizations must do more than suddenly show up one day at their community center, saying “let’s be friends, here’s a trial for you.”
Rather, it requires a steady presence that slowly builds a trusted relationship— and while most people understand this intellectually, not all follow through.
University of Florida’s Clinical and Translational Science Institute Assistant Director for Clinical Research and Chair, Developing Community and Culture Competence Council, Tiffany Danielle Chisholm Pineda launched a program in 2019 to increase cultural competence, community, and access in clinical research. The multifaceted Bright Voices program includes a range of initiatives to help create trusted relationships with historically underrepresented communities, including the “Black Voices in Research Storytelling Series,” a community of Black clinical research professionals who came together to share their experiences and offer ways to expand access to healthcare. Pineda’s successful program has expanded to other academic research institutions, including Ohio State University.
3. Foster Continued Diversity-Mindedness In Clinical Research Teams
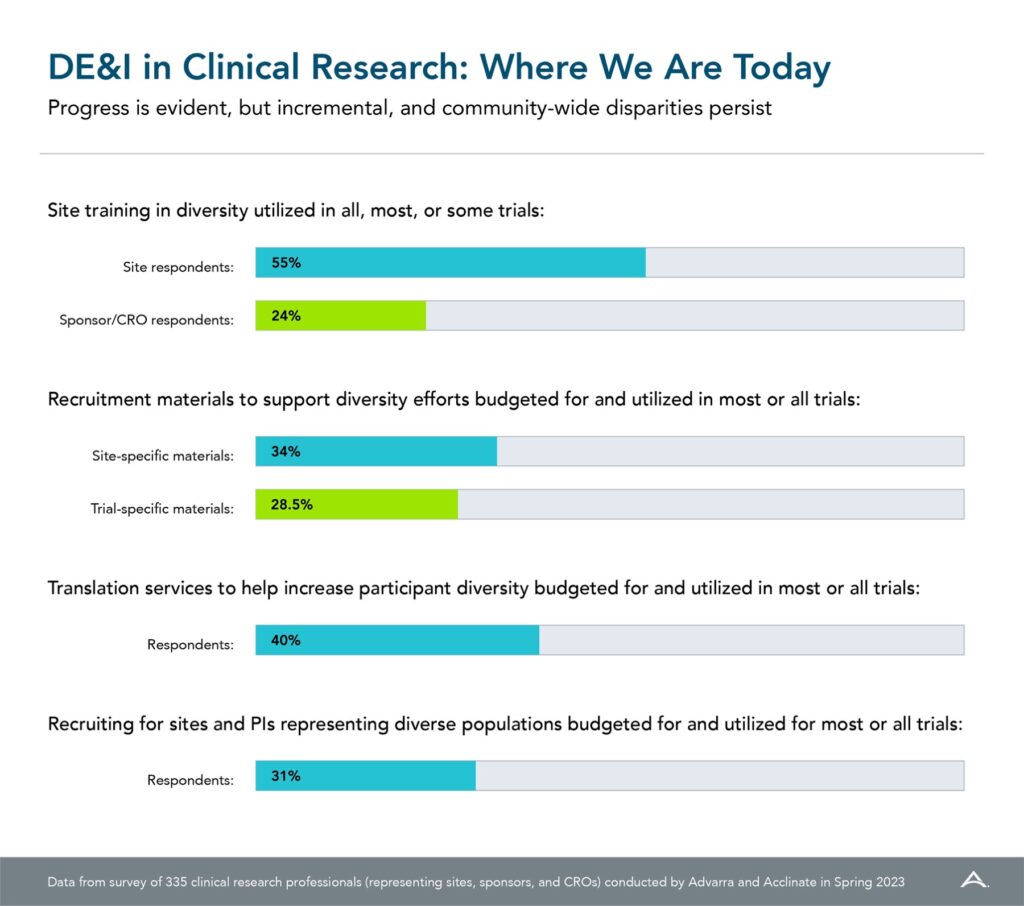
Just over half of sites engage in diversity training for their trials, according to a new survey report10 “DE&I in Clinical Research: Where Are We Now,” while only 34% of sponsors say they provide site training in diversity for all, most, or some trials. Given the emphasis on diversity in clinical research prompted by COVID-19 vaccine trial issues, these numbers are woefully low but suggest an area for improvement (Figure. 1). To foster greater participation across all patient populations, diversity-sensitivity and cultural-humility training are critical and should be tailored for different organizations. Sites and sponsors should adopt internal training programs, especially for individuals developing protocols or planning recruitment campaigns. Further, sponsors would benefit from providing DE&I training modules to participating sites, rather than just instructing sites to enroll diverse patient populations. Sites don’t always know the best ways to implement such directives.
“Training needs to be both study specific as well as more general, with guidance on how to build sustained community relationships,” explained Pope. “Sponsors and CROs should consider providing additional support and guidance for sites to help them understand what community engagement means and generate ideas for how to partner with the community.”
While the industry is moving in the right direction, racial and ethnic minorities still only account for 2% to 16% of clinical trial participants, while comprising 39% of the U.S. population.
In addition to training, organizations should also incorporate a spirit of diversity when hiring. Less than one- third (31%) of survey respondents make this a focus. It is important to recruit sites located in diverse communities, with staff and investigators from backgrounds representative of their communities to make participants feel more comfortable and create trust. This is also critical to help ensure a more representative and inclusive workforce for years to come.
4. Adopt New Participant Compensation and Engagement Approaches
It is important to offer different types of compensation methods and avoid broad assumptions based on unconscious bias—for instance, not all participants may have a bank account or rely on electronic banking providers such as Venmo or PayPal.
Stakeholders should also consider other ways to compensate participants and help ease the burden of participation. Travel reimbursement or providing childcare could help more people participate, as could offering flexible appointment hours. Helping potential participants with the economics of study participation may help increase enrollment as well as retention.
Additionally, using multiple recruitment approaches can help reach broader audiences. Not everyone will hear a radio ad, and some communities may be more receptive to a direct mailing or a social media post. One size does not fit all—a diversified recruitment plan can help reach more diverse participants.
“More accessible clinical research begins 64 PM360 MAGAZINE / JANUARY/FEBRUARY 2024
with equitable compensation methods and flexible support systems for participants,” said Parexel’s Rosamund Round, Vice President, Patient Engagement. “By recognizing the unique needs of patients and tailoring engagement approaches to different communities, we can bridge the gap and unlock a brighter future where innovative treatment options are far more accessible to patients.”
5. Carefully Address Cultural-Linguistic Barriers Prior to Patient Engagement
Many marginalized populations are suspicious of clinical trials—and not without good reason, given the past horrors of the Hepatitis Trial at Willowbrook11, USPHS Syphilis Study at Tuskeegee12, and the Havasupai Genetics13 case. It’s important to engage credible investigators who are members of the intended population and understand their culture and language.
While many sites and sponsors think they are taking into consideration cultural- linguistic barriers, many efforts are not nuanced enough to appeal to their target patient population. For instance, University of Florida’s Pineda highlights this issue in a tuberculosis trial focused on Haitian patients. “The trial could not get the enrollment numbers required. When I looked at the consent forms and other documents, I realized that they were translated into Creole rather than Haitian Creole. Just making this change helped enrollment,” said Pineda.
Put Your Money Where Your Mouth Is Moving forward, even with the greatest intentions, it’s critical that we focus more on population representation because that is what science dictates. If the industry wants to make real changes, it needs to make commensurate investment—and that includes investing in historically marginalized communities to demonstrate genuine commitment. Track performance metrics on those investments, and when there is positive return, let it inspire ongoing financing so the cycle of improvement continues.