Perspective is the capacity to understand the true relationship between ideas, outcomes and people. Of course, this is easier said than done. In 1969, Joni Mitchell popularized the notion while revealing how difficult it can be:
“I’ve looked at clouds from both sides now
From up and down, and still somehow
It’s cloud illusions I recall
I really don’t know clouds at all.”
Perspective, therefore, is not only recognizing that every coin has two sides but also understanding the relationship between those sides. The same can be said for delivering value in healthcare, which must encompass the widely varying perspectives of multiple stakeholders, each with a goal or approach that can dramatically affect that of the other.
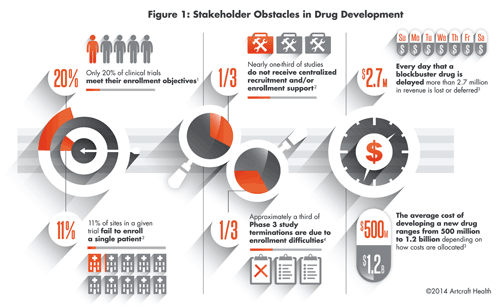
For example, the success of a clinical trial depends on understanding the perspectives of the enrolled participants, investigators, physicians and study sites. The underlying consideration must then be that the impact of one perspective, whether applied to the interpretation of data, the comprehension of information, or a stakeholder’s motivations, can generate a positive or negative outcome. Consider the bigger picture (see Figure 1).
Just consider that one third of clinical trials do not receive centralized recruitment/enrollment support, and only 20% of clinical trials meet their enrollment objectives. What can we correlate from this? That the success of a clinical trial and ultimate market launch is predicated, in no small measure, on the clinical trial’s success in engaging, informing, and motivating all of its stakeholders—patients, HCPs, and study sites alike.
Meeting Expectations
Consider the perspectives of the HCP, the patient and the site educator. How do you meet each one’s expectations? Easy: Remove fear, increase comprehension and awareness, make it clear and speak to their needs.
- Bring awareness to the point of care: A peer-to-peer letter can be effective in engaging HCPs in supporting the goals of a clinical trial. In many cases, the HCP is the first line of communication with the patient. By leveraging and providing targeted education to HCPs, you can increase enrollment and gain their support.
- Speak to the patient on his or her level: All too often, a patient’s expectations of a clinical trial are skewed. A 16-page informed consent form can say what needs to be said, but the patient who reads it, and may likely read at a 6th-grade level, takes from it a completely different understanding. All educational and informational solutions and materials intended for or directed to patients should be designed to be clear, actionable, relevant and engaging. If patients understand the expectations, they are likely to enroll, remain in the study, and adhere to and comply with the protocol.
- Provide education for the educators: Study-site coordinators, who are generally tasked with educating potential subjects about a clinical trial and enrolling them, are sometimes unaware of a potential subject’s emotional state or level of health literacy, or they may have insufficient resources to perform those tasks. Give the site coordinators the educational solutions to engage patients and approach them on their level. Remove the “white binder syndrome” and create an identity and “feel” that brands your trial. It will keep your trial top-of-mind with the sites, and they will thank you for making their job easier.
Improve Your Bottom Line
Without education, awareness and targeted support directed to all phases of product development, from the recruitment of a clinical trial to a product’s market entrance, the money and time spent on these efforts—and the opportunity to bring a potentially lifesaving treatment to a patient—will be delayed or lost. This is a bold statement, but in our experience, there is a distinct correlation.
Once upon a time, there was a long held belief that “if you build it, they will come.” The premise of this belief was not unwarranted, given the historical success of clinical trials and the postmarket performance of approved drugs. However, that time has passed, and it’s no longer just about the right protocol and the right investigator.
When it comes to the ability to deliver a treatment for a patient in need, the most important perspective to consider is that of the patient. Without patients, clinical research doesn’t happen, and without clinical research, a drug does not go to market. So consider this when planning for your trial. What’s your patient perspective?
References:
1. Hess J; “Web-based Patient Recruitment: Best Opportunity To Accelerate Clinical Trials.” Cutting Edge Information. http://www.cuttingedgeinfo.com/process/?ref=122. Accessed August 19, 2014. Registration required.
2. “Impact Report: Analysis and Insight into Critical Drug Development Issues. 89% of Trials Meet Enrollment, But Timelines Slip, Half of Sites Under-enroll.” Tufts Center for the Study of Drug Development. http://csdd.tufts.edu/files/uploads/jan-feb_2013_ir_summary.pdf. Accessed August 19, 2014.
3. Snee RD; “Achieving More Efficient and Effective Clinical Trials: How To Create and Sustain Improvements in Critical Work Processes.” Tunnel Consulting. http://tunnellgov.com/docs/resources/Tunnell-Government-Whitepaper_Clinical-Trials.pdf. Accessed August 19, 2014.
4. Li G, Gray R. “Performance-based Site Selection Reduces Costs and Shortens Enrollment Time.” The Monitor, December 2011.