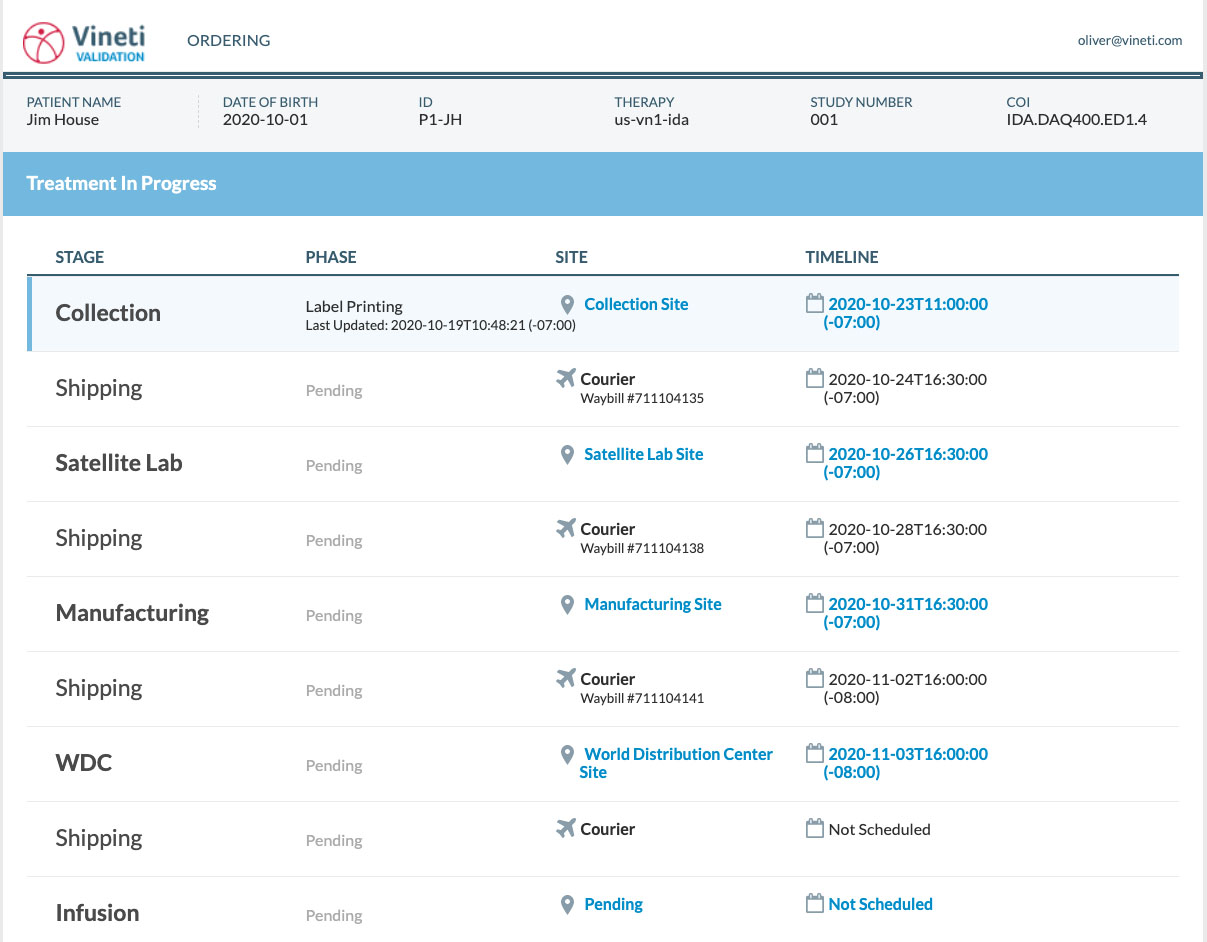
Personalized medicine—such as cell and gene therapies (CGT) and personalized cancer vaccines—are completely changing how therapies are manufactured. These medications are often tailored to an individual patient’s molecular information and in some cases are even made using a patient’s or donor’s cells. That means samples have to be taken from a patient or donor, re-engineered in a lab, and then infused into the right patient. The process can involve several stops along the supply chain, strict quality and safety requirements, and special shipping processes—all of which is done for a single batch for one patient.
Recognizing the unprecedented challenges this would present to the supply chain, the team at Vineti developed a solution to make things possible for everyone involved. PM360 spoke with Chief Executive Officer and Co-founder Amy DuRoss about how the company is working to streamline the process for personalized medicine, the issues that must be overcome to help scale the production of these therapies, and what the future holds for this market.

PM360: How did this company originally get off the ground?
Amy DuRoss: We began as a response to a problem statement Novartis put out almost seven and a half years ago when the company was looking at the commercialization pathway for their CAR-T therapy, Kymriah, that was in Phase 3 at that time. Basically, the question was this: How do you scale out this incredibly personalized, complex production and delivery process?
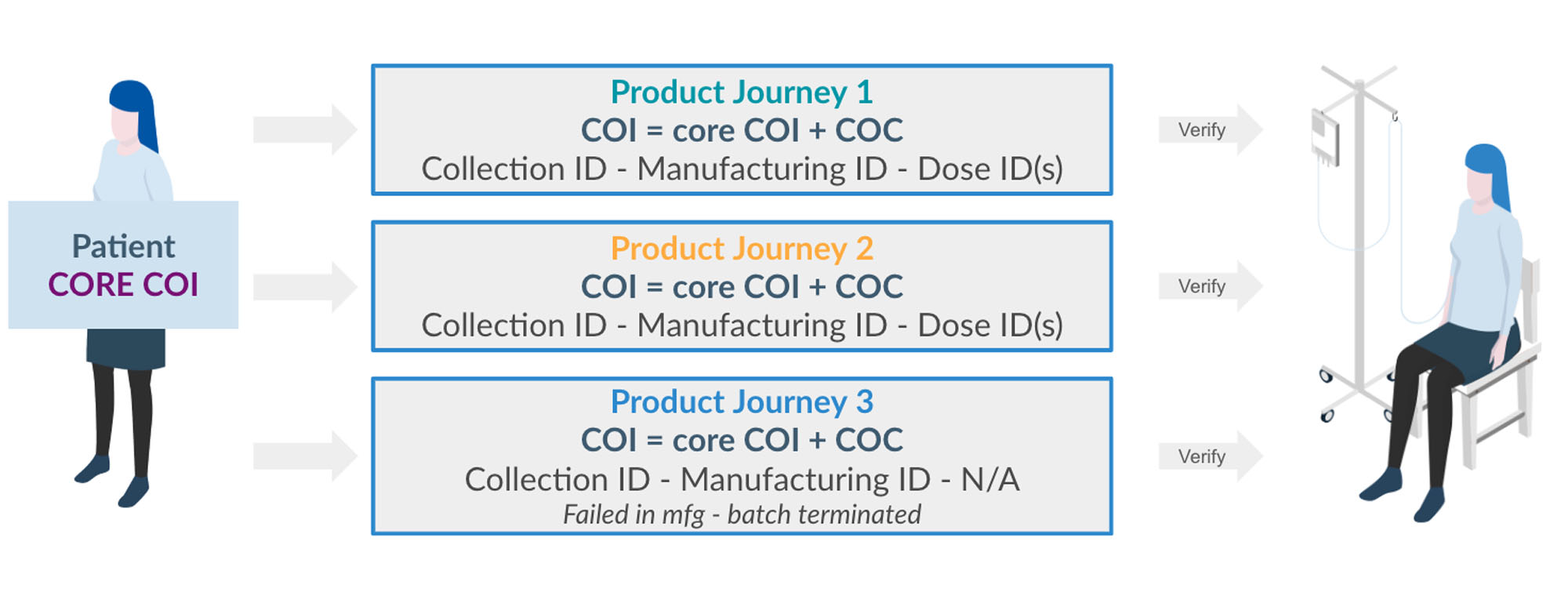
At the time, I was Managing Director of the Ventures team at GE where we were already examining both investments related to personalized medicine as well as opportunities to create focused, independent new companies that would address key gaps in the personalized therapy market. We went through about 18 months of diligence with the Mayo Clinic as the co-founding partner and participation from key pharma industry leaders, patients, providers, regulators, and logistical providers—all aspects of the supply chain. We discovered that one of the key missing links to achieve industrialization in CGT was a software platform that would digitize Chain of Identity (COI) and Chain of Custody (COC) so that each single batch production would have a unique patient identifier and a way to essentially assume that the right patient would always receive the right therapy at the right time.
How does your platform work? Do all parties along the supply chain have to use it?
Our software system, which we call the Personalized Therapy Management (PTM) platform, enables biopharma companies to manage complex compliance, scheduling, logistics, and all of the interactions they need to have with clinical partners, manufacturing partners, suppliers, and logistical partners in a single source of truth. The system is built on best practice enterprise software precepts with a set of fully configurable modules on top of the infrastructure.
 You are not required to use all of the modules on our platform. We deploy the modules that are relevant to the protocols you are supporting. For example, you may have a product that won’t actually need to be dependent on source material in the clinic, but rather will draw from a donor database of other cells. In that case, you wouldn’t need to interface directly into a clinical setting until you’re administering a patient with a quality assurance product or therapeutic.
You are not required to use all of the modules on our platform. We deploy the modules that are relevant to the protocols you are supporting. For example, you may have a product that won’t actually need to be dependent on source material in the clinic, but rather will draw from a donor database of other cells. In that case, you wouldn’t need to interface directly into a clinical setting until you’re administering a patient with a quality assurance product or therapeutic.
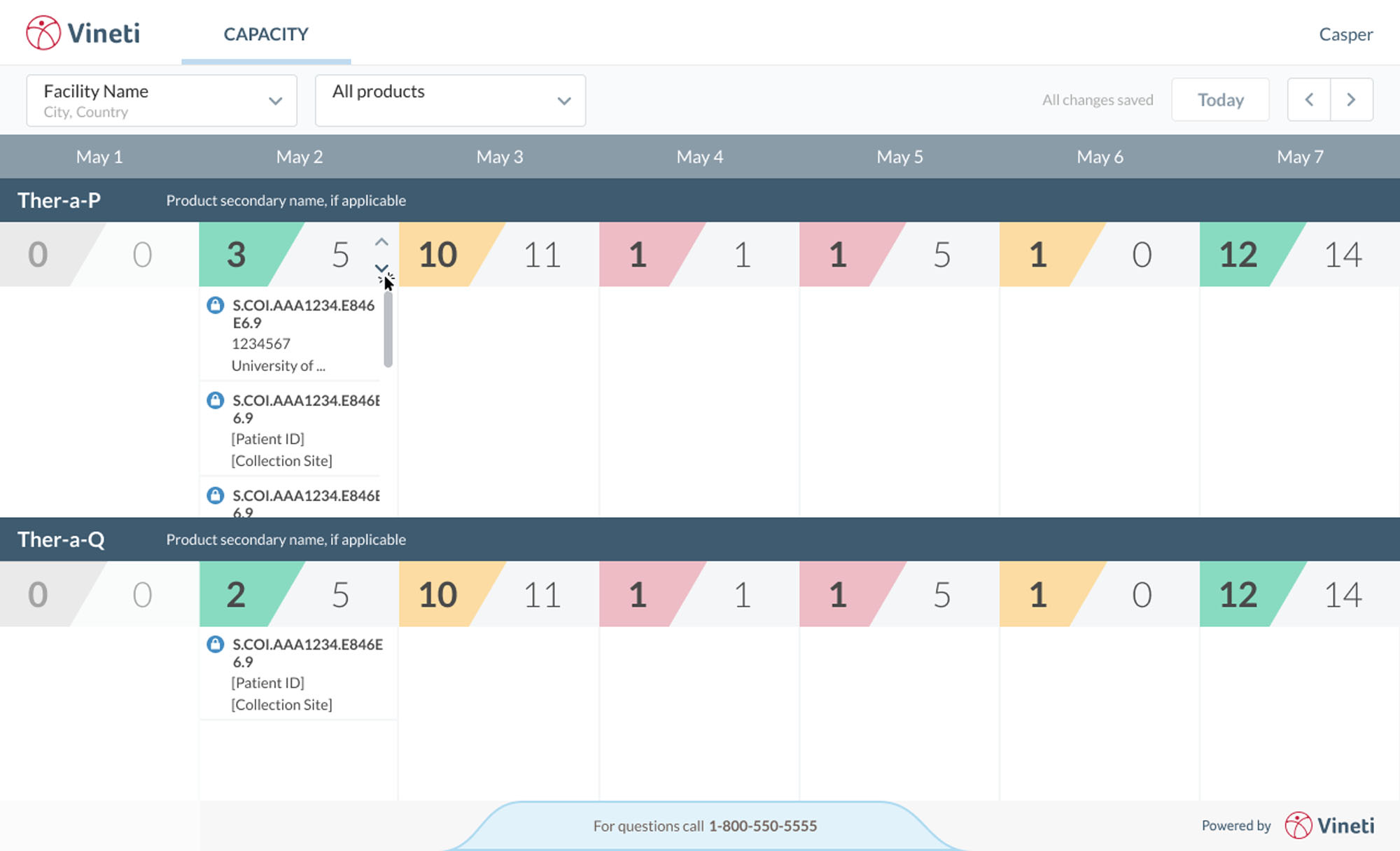
Additionally, our system is built to adjust to a market in which the science, processes, and regulations are constantly evolving. We’re just in the early innings of this whole marketplace, so we needed to ensure we built an infrastructure capable of keeping up with change.
As you have been implementing this across the supply chain, what’s been the biggest challenge?
This whole area of medicine is shifting a paradigm and causing parts of the healthcare ecosystem to interact in a dependency that they never had to in the past. That means we now need the specialty couriers, cold chain providers, hub managers, care pathway managers, clinical personnel, manufacturing personnel, and even anyone who’s managing reimbursement to all adhere to a single process with a single set of workflows. So the biggest challenge has been creating a common vernacular across all of these parties to achieve that alignment. But that has started to really ameliorate, and the end rewards are so great because once you get alignment, then you actually increase efficiency for everybody.
As you work with companies using your platform, what areas have you found to be still in need of refinement?
So many things. There’s a tremendous amount of humility coming to this marketplace because the complexity is enormous. For example, the more we dig into COI and COC, the more complexity we find and the more opportunities we uncover.
One reason for that is COI and COC doesn’t stop at the point of infusion. Patients may need to be monitored, ostensibly, the rest of their lives. Reimbursement is another consideration, because if these products have value contracts, then checkpoints along the way must be measured for efficacy, so we need to be able to add that to the compliance report we produce for every single product. This would also allow us to better compile real-world evidence and have an opportunity to recycle appropriate learnings for future discovery and stratification of patients for future trials.
Considering this form of medicine is so new, what do you see as Vineti’s role five or 10 years down the road once these therapies are more established and the processes are more standardized?
We have a unique vantage point on the pipeline and the science is flabbergasting. So, first and foremost, I am just excited there are going to be real life-changing and life-saving options on the market. Five to 10 years from now I expect we should have 40 to 50 products out and at least 10,000 in discovery at various phases—and that may be conservative. These new therapies are going to be a mix of what we’re seeing today, including gene modified, autologous, allogeneic, and cancer vaccines, as well as even more advancements due to the horsepower of CRISPR technologies.
If Vineti accomplished our goal, we will have expanded access to many more patients for those therapies by establishing a level of network orchestration that goes beyond just standardization and achieves optimization. So our goal is to recycle our learnings as quickly as we can, invest in the horsepower of our platform, and take on as many use cases and partners as possible so we can help achieve the mass customization of these therapies.







