AT ACC 16
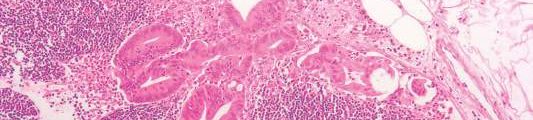
CHICAGO (FRONTLINE MEDICAL NEWS) – The incidence of valve hemodynamic deterioration in the first year after transcatheter aortic valve replacement is about 2.5%, but this event wasn’t clearly associated with adverse clinical outcomes out to 18 months of follow-up in an analysis of the large U.S. registry collaboratively maintained by the Society of Thoracic Surgeons and the American College of Cardiology.
“These findings, especially the patient and procedural predictors of valve hemodynamic deterioration we identified, may help to inform TAVR care, including patient selection, surveillance, and preventive strategies,” Dr. Sreekanth Vemulapalli reported at the annual meeting of the American College of Cardiology.
Recent reports have linked TAVR to subsequent development of leaflet abnormalities and valve thrombosis, with widely ranging estimates of incidence. Definitive answers as to the true rate of these adverse events and the underlying mechanisms will come from ongoing prospective studies using advanced imaging via four-dimensional CT or transesophageal echocardiography, but those studies will take years to complete, noted Dr. Vemulapalli of the Duke Clinical Research Institute in Durham, N.C.
In the meantime, he continued, the STS/ACC Transcatheter Valve Therapy Registry provides a unique opportunity to shed light on the incidence and consequences of valve hemodynamic deterioration (VHD) in real-world clinical practice. The registry includes all commercial TAVR procedures performed in the United States, with transthoracic echocardiograms obtained pre- and post-TAVR, at 30 days, and at 1 year after the procedure.
To examine the short- and longer-term rates of VHD, which Dr. Vemulapalli and his coinvestigators defined as an increase in the mean aortic valve gradient of 10 mm or more, the researchers assembled two separate patient cohorts. They comprised a short-term–risk group of 10,095 patients who underwent TAVR at 334 sites, with an incidence of VHD of 2.1% during the first 30 days after the procedure, and 3,175 patients at 254 sites, whose incidence of VHD from day 30 through 1 year post TAVR was 2.5%.
The combined rate of VHD and all-cause mortality during the first 30 days was 7.1%. For the long-term cohort, the combined endpoint rate from day 30 to 1 year was 23.5%.
Importantly, the occurrence of VHD was not associated with an excess of the composite endpoint of mortality, stroke, heart failure hospitalization, or aortic valve reintervention at 1 year in either the short- or long-term cohort. The same held true in an analysis covering the period of 12-18 months post TAVR, according to Dr. Vemulapalli.
In a multivariate analysis, the significant predictors of VHD in the short-term cohort were male sex; increased body mass index, with the risk rising stepwise with every additional 5 kg/m above normal weight; baseline severe chronic lung disease; a valve-in-valve procedure; a larger baseline aortic valve gradient; a TAVR valve size of 23 mm or less; and severe patient/prosthesis mismatch.
In the long-term cohort, the risk factors for VHD were hospital discharge on a factor Xa inhibitor and a larger predischarge aortic valve gradient.
Change in left ventricular ejection fraction over the course of the study bore no relation to VHD risk. Neither did which of the two commercially available TAVR valves a patient received.
This study was funded by the American College of Cardiology’s National Cardiovascular Data Registry. Dr. Vemulapalli reported serving as a consultant to Novella and Premiere and receiving research grants from the Agency for Healthcare Research and Quality, Boston Scientific, Abbott Vascular, and the ACC.