FROM JAMA
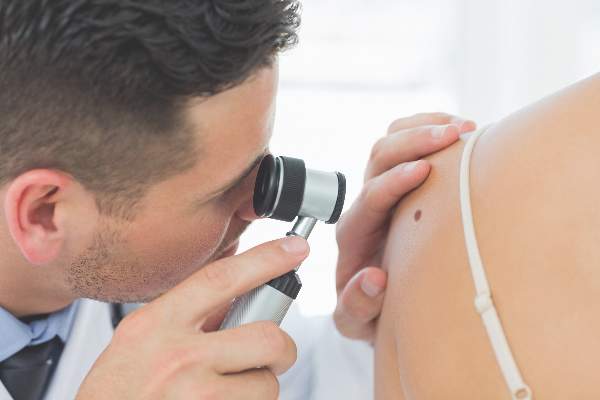
The benefits and harms of visual screen cancer screening exams for asymptomatic adults can’t be adequately assessed with current evidence, according to a new recommendation from the U.S. Preventive Services Task Force.
“Evidence is inadequate to reliably conclude that early detection of skin cancer through visual skin examination by a clinician reduces morbidity or mortality,” according to the statement published online July 26 in JAMA ( 2016;316[4]:429-435. doi:10.1001/jama.2016.8465 ).
Approximately 76,400 adults in the United States will develop melanoma, and more than 10,000 will die from it, according to the USPSTF. However, more than 98% of skin cancer cases in the United States are basal and squamous cell carcinoma, which have much lower morbidity and mortality rates, noted the USPSTF researchers, led by Kirsten Bibbins-Domingo, MD, PhD, of the University of California, San Francisco.
The current statement updates the USPSTF’s 2009 recommendation, which also found insufficient evidence to assess the harms and benefits of visual skin cancer screening in asymptomatic adults with no history of premalignant or malignant skin lesions. However, the current recommendation eliminates a statement about patients’ skin self-exams.
According to the USPSTF, evidence is “adequate” that a clinician’s visual skin exam has “modest sensitivity and specificity for detecting melanoma,” but evidence is inconsistent to support the ability of a visual skin exam to detect nonmelanoma skin cancer.
The USPSTF commissioned an evidence review that included 11 studies previously reviewed and 2 additional studies conducted since 2009. The two new studies included one that evaluated skin cancer screening performed by dermatologists or plastic surgeons and one that evaluated skin cancer screening performed by primary care physicians. Sensitivity and specificity in the two studies ranged from 40% to 70% and from 86% to 98%, respectively.
“None of the studies could draw reliable conclusions as to whether screening performed by any of the clinical specialties differed in diagnostic accuracy,” the researchers noted. In addition, “no [randomized controlled trial] has directly evaluated the effectiveness of the clinical visual skin examination for reducing skin cancer morbidity and mortality,” they wrote.
The recommendation was accompanied by several editorials published online July 26 in JAMA journals.
In JAMA, Hensin Tsao, MD, PhD, of Massachusetts General Hospital, Boston, and Martin Weinstock, MD, PhD, of Brown University, Providence, R.I., noted that the USPSTF considered the possibility of including information from high-quality case-control studies in lieu of randomized controlled trials, which have been difficult to conduct in skin cancer screening. “The evidentiary standard needs to be further refined to be appropriate to the modest magnitude of potential harms of a properly performed skin cancer screening,” they wrote (JAMA. 2016;316:398-400). Dr. Tsao disclosed an honorarium from Lubax.
In JAMA Dermatology, Susan Swetter, MD, of the Veterans Affairs Palo Alto (Calif.) Health Care System; Alan C. Geller, MPH, of Harvard School of Public Health, Boston; and Allan C. Halpern, MD, of Memorial Sloan Kettering Cancer Center, New York, wrote about ways to promote broader uptake of skin cancer screening. “Alternative models should be explored to bundle skin screening with other preventive services (e.g., blood pressure measurements or flu shots) and to engage advanced practice providers (e.g., nurse practitioners and physician assistants) to promote screening among individuals with less access to dermatologists,” they wrote (JAMA Dermatol. 2016. doi: 10.1001/jamadermatol.2016.2606).
In JAMA Oncology, Vinayak K. Nahar, MD, of the University of Mississippi Medical Center, Jackson; Jonathan E. Mayer, MD, of Johns Hopkins University, Baltimore; and Jane M. Grant-Kels, MD, of the University of Connecticut, Farmington, addressed concerns over performing more biopsies. “The USPSTF also raises concern over the number needed to biopsy to detect 1 case of melanoma. In weighing these data, one must also consider that many of the nonmelanomas biopsied were likely severely atypical nevi that have their own risk of malignant transformation. Although difficult to quantify, there is some benefit to removing a severely atypical nevus, both for risk of transformation and for a patient’s peace of mind,” they wrote (JAMA Oncol. 2016. doi: 10.1001/jamaoncol.2016.2440).
In JAMA Internal Medicine, Eleni Linos, MD, of the University of California, San Francisco; Kenneth A. Katz, MD, of Kaiser Permanente, San Francisco; and Graham A. Colditz, MD, of Washington University, St. Louis, cautioned that the USPSTF recommendations shouldn’t be interpreted as minimizing the importance of skin cancer. “Instead, the report should motivate us to improve the evidence base for identifying groups of people in whom the benefits of screening might outweigh risks,” they wrote. “Meanwhile, we should also fully implement skin cancer primary prevention by eliminating indoor tanning exposure, especially among youths, and increasing the use of sun-protection strategies that work” (JAMA Intern. Med. 2016. doi: 10.1001/jamaintermed.2016.5008).
The recommendations are not an official position of the U.S. Department of Health and Human Services or the Agency for Healthcare Research and Quality.